Abstract
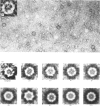
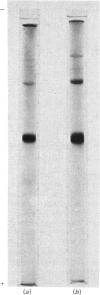
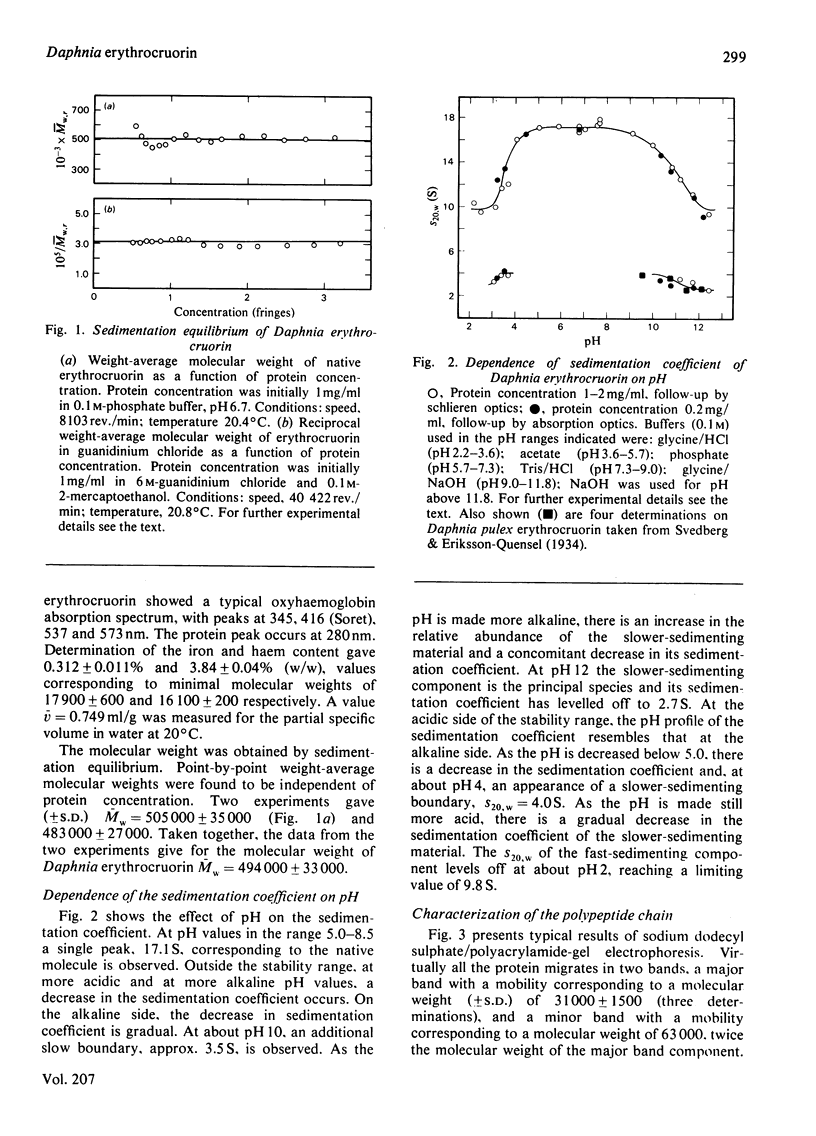
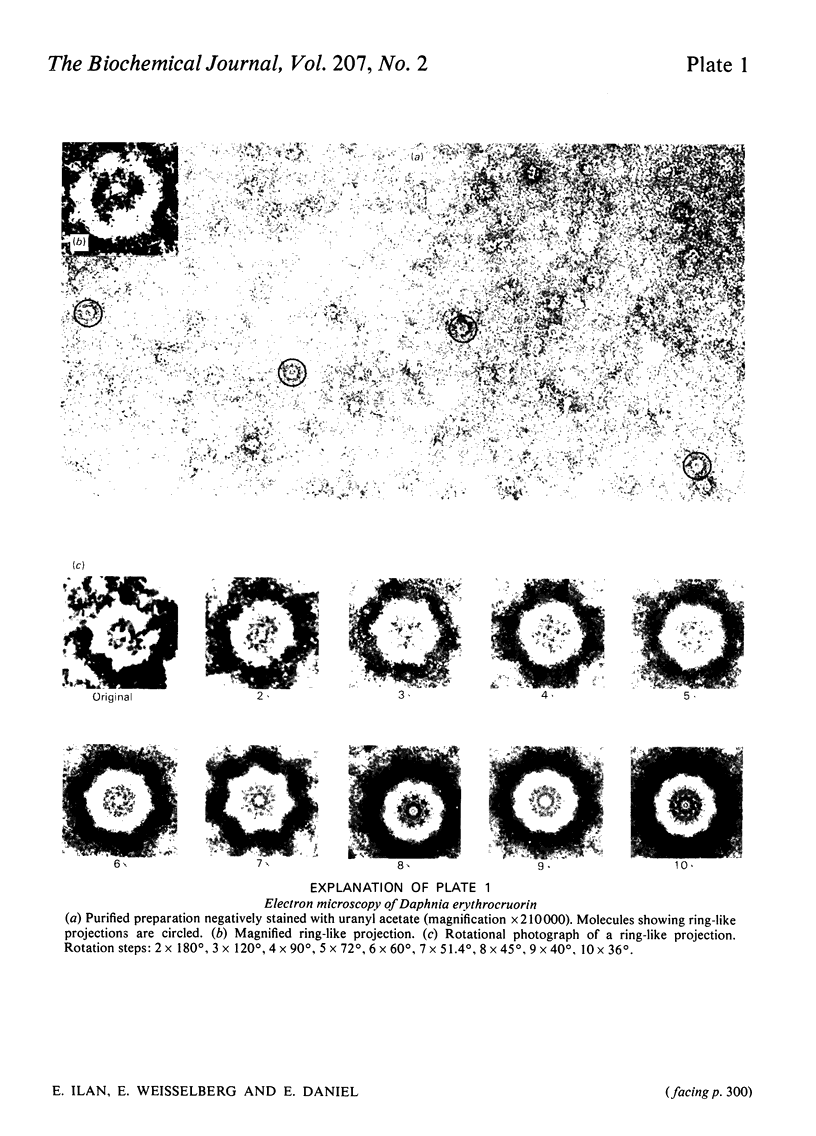
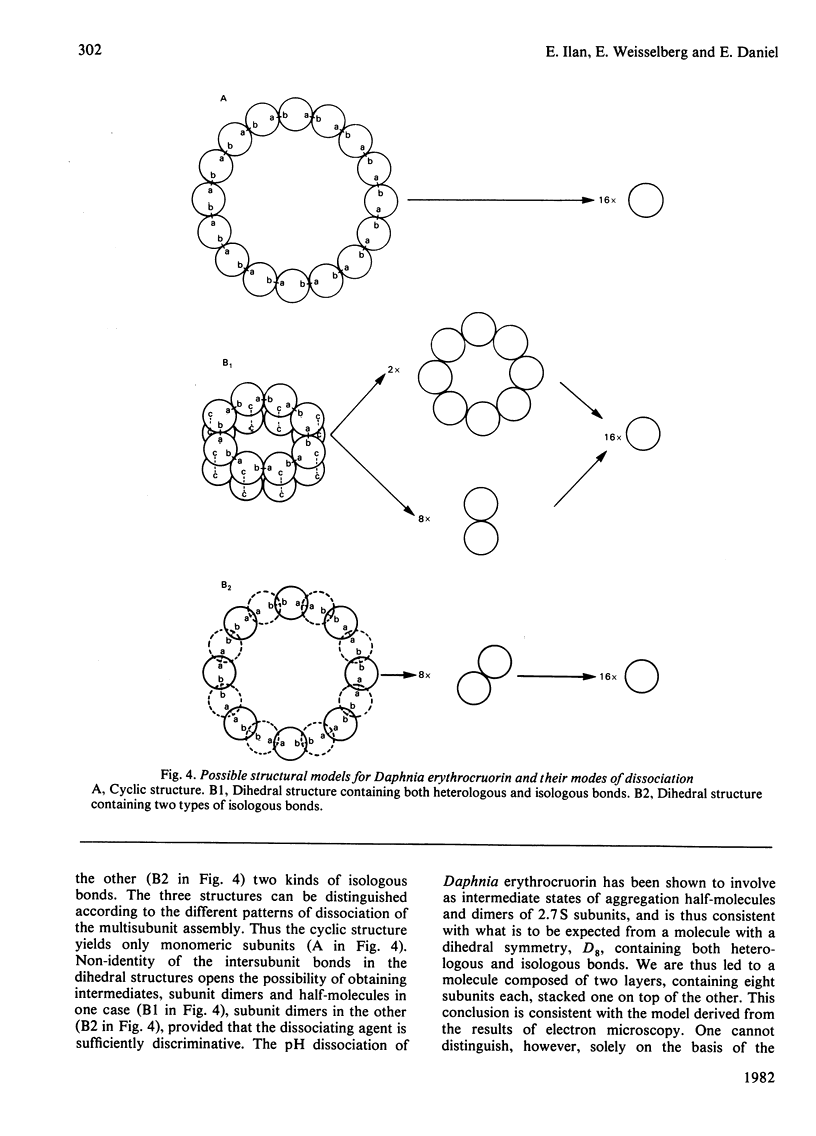
The subunit structure of erythrocruorin from the cladoceran Daphnia magna was studied. The native protein was found to have a sedimentation coefficient (S2(20), w) of 17.9 +/- 0.2 S and a molecular weight, as determined by sedimentation equilibrium, of 494 000 +/- 33 000. Iron and haem determinations gave 0.312 +/- 0.011% and 3.84 +/- 0.04%, corresponding to minimal molecular weights of 17900 +/- 600 and 16 100 +/- 200 respectively. Sodium dodecyl sulphate/polyacrylamide-gel electrophoresis gave one band with mobility corresponding to a molecular weight of 31 000 +/- 1 500. The molecular weight of the polypeptide chain determined by sedimentation equilibrium in 6 M-guanidinium chloride and 0.1 M-2-mercaptoethanol is 31 100 +/- 1300. On a molecular-weight basis, Daphnia erythrocruorin is composed of 16 identical polypeptide chains carrying two haem groups each. The native structure is stable between pH5 and 8.5. At alkaline and acidic pH, a gradual decrease in the sedimentation coefficient down to 9.8S occurs. Above pH 10 and below pH4, a slow component with S20, w between 2.7S and 4.0S is observed. The 2.7S, 4.0S and 9.8S species are identified as single-chain subunits, subunit dimers and half-molecules respectively. We propose a model for the molecule composed of 16 2.7S subunits grouped in two layers stacked in an eclipsed orientation, the eight subunits of each layer occupying the vertices of a regular eight-sided polygon. Support for this arrangement is provided from electron microscopy and from analysis of the pH-dissociation pattern.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CASASSA E. F., EISENBERG H. THERMODYNAMIC ANALYSIS OF MULTICOMPONENT SOLUTIONS. Adv Protein Chem. 1964;19:287–395. doi: 10.1016/s0065-3233(08)60191-6. [DOI] [PubMed] [Google Scholar]
- FOX H. M. Haemoglobin in the Crustacea. Nature. 1957 Jan 19;179(4551):148–148. doi: 10.1038/179148a0. [DOI] [PubMed] [Google Scholar]
- Ilan E., Daniel E. Haemoglobin from the tadpole shrimp, Lepidurus apus lubbocki Characterization of the molecule and determination of the number of polypeptide chains. Biochem J. 1979 Nov 1;183(2):325–330. doi: 10.1042/bj1830325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan E., David M. M., Daniel E. Erythrocruorin from the crustacean Caenestheria inopinata. Quaternary structure and arrangement of subunits. Biochemistry. 1981 Oct 13;20(21):6190–6194. doi: 10.1021/bi00524a043. [DOI] [PubMed] [Google Scholar]
- Klotz I. M., Langerman N. R., Darnall D. W. Quaternary structure of proteins. Annu Rev Biochem. 1970;39:25–62. doi: 10.1146/annurev.bi.39.070170.000325. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Timasheff S. N. The calculation of partial specific volumes of proteins in 6 M guanidine hydrochloride. Methods Enzymol. 1979;61:49–57. doi: 10.1016/0076-6879(79)61006-6. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Matthews B. W., Bernhard S. A. Structure and symmetry of oligomeric enzymes. Annu Rev Biophys Bioeng. 1973;4:257–317. doi: 10.1146/annurev.bb.02.060173.001353. [DOI] [PubMed] [Google Scholar]
- McMEEKIN T. L., MARSHALL K. Specific volumes of proteins and the relationship to their amino acid contents. Science. 1952 Aug 8;116(3006):142–143. doi: 10.1126/science.116.3006.142. [DOI] [PubMed] [Google Scholar]
- Shlom J. M., Vinogradov S. N. A study of the subunit structure of the extracellular hemoglobin of Lumbricus terrestris. J Biol Chem. 1973 Nov 25;248(22):7904–7912. [PubMed] [Google Scholar]
- Sugano H., Hoshi T. Purification and properties of blood hemoglobin from the fresh-water cladocera, Moina macrocopa and Daphnia magna. Biochim Biophys Acta. 1971 Feb 16;229(2):349–358. doi: 10.1016/0005-2795(71)90194-2. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Wood E. J., Mosby L. J. Physicochemical properties of Planorbis corneus erythrocruorin. Biochem J. 1975 Aug;149(2):437–445. doi: 10.1042/bj1490437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]