Figure 9.
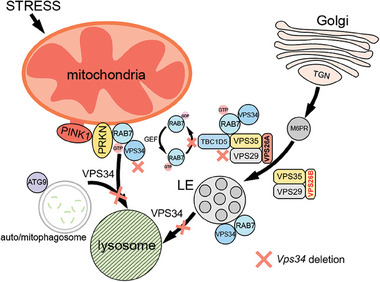
A schematic diagram illustrates the functional mechanism of VPS34 in oocytes. The critical role of VPS34 in oocyte is manifested by the blockage of mitophagy and autophagy flux in both ZcKO oocytes and derived embryos. VPS34 is also crucial for regulating RAB7 activity and facilitating its translocation on mitochondrial membrane during mitophagy initiation, as well as for the RAB7‐mediated ATG9 trafficking during mito/autophagosome formation. The RAB7 GAP TBC1D5, in complex with VPS26A retromer, regulates the activity of RAB7 by converting it from GTP to GDP state. The regulatory process is reliant on the direct interaction between RAB7 and VPS34. When VPS34 is deleted, it leads to an increased expression of VPS26B. VPS26B then competes with VPS26A to form an alternative retromer complex, which consequently reduces the interactions between TBC1D5 and RAB7. As a result, there is an increase in RAB7 activity. When RAB7 is hyperactivated, it is unable to properly translocate to the mitochondria membrane, leading to a failure in forming ATG9‐mediated mito/autophagosome formation during PRKN‐mediated mitophagy. Thus, VPS34 is not only involved in the maturation of late endosomes and lysosomes but also plays a crucial role in initiating mitophagy by regulating the activity and subcellular location of RAB7. In ZcKO oocytes, the accumulation of mitochondrial damage and blockage of autophagy ultimately result in impaired oocyte developmental competence.
