Abstract
Although microvascular decompression (MVD) is a reliable treatment for hemifacial spasm (HFS), postoperative delayed relief is one of its main issues. We previously evaluated the morphology of the lateral spread response (LSR) and reported correlation between delayed relief after MVD and polyphasic morphology of the LSR. This study aimed to investigate the morphology of LSR and the course of recovery of the compound motor action potential (CMAP), to better understand the pathophysiology of delayed healing of HFS. Based on the pattern of the initial LSR morphology on temporal and marginal mandibular branches stimulation, patients were divided into two groups: the monophasic and polyphasic groups. The results of MVD surgery and sequential changes in the CMAP were evaluated 1 week, 1 month, 1 year, and final follow-up after the surgery. Significantly higher rates of persistent postoperative HFS were observed in patients with the polyphasic type of initial LSR at 1 week and 1 month after the surgery (P < 0.05, respectively). In the polyphasic group, the amplitude of the CMAP tended to gradually improve with time, while in the monophasic group, the amplitude of the CMAP decreased on the seventh postoperative day, followed by its gradual improvement. There is a significant correlation between delayed relief after MVD and polyphasic morphology of the initial LSR in patients with HFS. In the polyphasic group, CMAP recovered earlier and showed less reduction in amplitude, suggesting segmental demyelination, with less damage to peripheral nerves.
Keywords: lateral spread response, demyelination, ephaptic transmission, hemifacial spasm, microvascular decompression
Introduction
Hemifacial spasm (HFS) is a motor disorder characterized by involuntary tonic-clonic activity of the muscles innervated by the facial nerve (FN) on the ipsilateral side of the face.1,2) Since vascular compression of the FN at the root exit zone (REZ) is widely accepted as the main cause of HFS,3-6) microvascular decompression (MVD) of the FN is a well-established surgical treatment for HFS, producing relatively good results with minimal complications.7-12) Although most of the patients immediately become spasm-free, approximately 10%-40% still experience residual spasm after MVD surgery.13-20)
Terasaka et al. revealed a significant correlation between preoperative anticonvulsant therapy and delayed cure after MVD in a multivariate analysis,19) and Sato et al. mentioned non-transposition as one of the causes of delayed relief in HFS,21) although the other causes of delayed symptom relief in HFS remain unclear.
Lateral spread responses (LSRs), elicited by electrically stimulating one branch of the FN while recording electromyographic responses from a muscle innervated by another branch of the FN, are useful for the electrophysiological diagnosis of HFS, because they represent an abnormal electromyographic response characteristic in HFS patients.22,23) So far, LSR waves have only been assessed as “residual” or “diminished” during MVD and have not been evaluated qualitatively.
We previously focused on the morphology of the LSR waveform and reported significant correlation between delayed relief after microvascular decompression and polyphasic morphology of the LSR in patients with HFS.24) To perform further qualitative evaluation, we investigated the relationship between the morphology of the LSR waveform and sequential long-term electrophysiological changes in motor function of the FN, evaluated using the compound motor action potential (CMAP), after MVD surgery. To our knowledge, this study is the first to examine the correlation between initial LSR morphology and sequential changes in CMAP after MVD in patients with HFS.
Materials and Methods
Study design
All participants provided informed consent, and the study protocol was approved by the Ethics Committee of Nakamura Memorial Hospital and was performed in accordance with the principles of the Declaration of Helsinki. Participants in this observational, nonrandomized study were identified via a retrospective electronic chart review of HFS patients treated by MVD between January 2015 and March 2020 at the Nakamura Memorial Hospital. All surgical procedures were performed by a senior doctor (N.S.), a skilled neurosurgeon with 23 years of experience.
Patients
To allow evaluation of the unaffected FN, patients who met the following criteria were excluded: (1) past medical history of botulinum neurotoxin injection, (2) previous MVD surgery, (3) presence of preoperative facial weakness, and (4) history of Bell's palsy, trauma, or other surgical treatment around the FN area. Patients with (5) an unmeasurable initial LSR or in whom all the waveform amplitudes were less than 10 μV and (6) early loss to CMAP follow-up were also excluded.
Intraoperative LSR monitoring
Intraoperative LSR monitoring during MVD surgery was performed using a Neuromaster MEE-1232 or a Neuromaster G1 MEE-2000 monitoring system (Nihon Kohden, Inc., Tokyo, Japan). After induction of general anesthesia, one needle was inserted into the orbicularis oculi muscle and the other into a subcutaneous electrically inactive site. For the mentalis muscle, electrodes were implanted in the same manner. All needles used a length of 0.4×13 mm. LSRs were recorded from the mentalis muscle after electrical stimulation of the temporal branch of the FN and from the orbicularis oculi muscle after stimulation of the marginal mandibular branch of the FN. LSRs were recorded using amplifiers with a frequency band of 20 to 3 kHz. The initial LSR was recorded by supramaximal stimulation before opening the dura mater, after confirming the depth of general anesthesia with bispectral index monitoring. The initial LSR was measured several times to confirm its repeatability. Subsequently, LSR was continuously recorded at 1-min intervals during surgery. Cases in which the LSR disappeared with stimulation of both the temporal and marginal mandibular branches were defined as showing LSR disappearance.
Stimulation and recording of CMAP
The nerve conduction study measurement was performed as described in a previous report.2,25) Briefly, the patients lay on a bed in the supine position in a warm room. The FNs were stimulated using a bipolar surface electrode with the cathode positioned below the ear lobe and the anode on the mastoid tip. The recording electrodes were disks 5 mm in diameter, placed on the inferior part of the orbicularis oculi muscle. The ground electrode was placed on the forehead. A square wave stimulus of 0.2 ms at 1 Hz frequency was used to generate the highest level of muscle action potential. We measured the CMAP amplitude on both sides of the face, and subsequently recorded the amplitude ratio of the affected and unaffected sides. A CareFusion Nicolet EDX with Viking Software system (Natus Neurology, Middleton, WI, USA) was used for the stimulations and measurements.
Evaluation of surgical results
The results of surgery for HFS were evaluated 1 week, 1 month, and 1 year and final follow up after the surgery. Based on the classification proposed by the Japan Society for Microvascular Decompression Surgery, the symptoms of HFS were evaluated as either being cured or not at the time of each follow-up. In this study, all follow-ups in the patients were conducted only by the senior doctor (N.S.).
Analysis of the initial LSR morphology
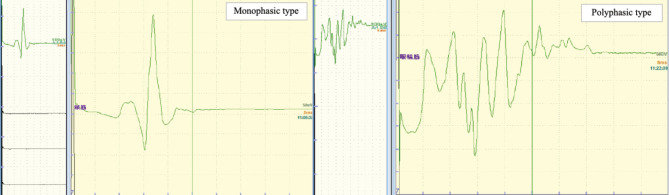
LSRs were classified based on their pattern as monophasic and polyphasic types (Fig. 1). Briefly, waveforms with up to two spikes of over 30% of the maximum amplitude were defined as the monophasic type, while those with three or more spikes were defined as the polyphasic type. The number of spikes, their duration, and the maximum amplitude of the initial LSR were measured and compared between the two types of waveforms. LSR morphology was analyzed by three experienced neurosurgeons blinded to the clinical data. In cases where there were discrepancies in the results of waveform evaluations, the majority decision was given priority.
Fig. 1.
Classification of the lateral spread response.
The patterns of the lateral spread response (LSR) were classified as monophasic and polyphasic types. Briefly, waveforms with up to two spikes over 30% of the maximum amplitude were defined as monophasic type and those with three or more spikes as polyphasic type.
Clinical and statistical analysis
Based on the results of the initial LSR morphology with orbicularis oculi and mentalis muscle stimulation, the patients were divided into two groups: patients with a monophasic spike pattern in the initial LSR for both muscles were categorized as the monophasic group and those with a polyphasic LSR pattern in even one of the muscles evaluated were classified as the polyphasic group.
The primary outcome was the amplitude ratio of the CMAP (CMAP affected side/CMAP unaffected side) preoperatively, and at 1 week, 1 month, and 1 year, after the surgery. Each group was also evaluated for baseline characteristics; healing rate of residual HFS at 1 week, 1 month, and 1 year and final follow up after the surgery; intraoperative LSR disappearance rate; and permanent complications rate.
Categorical variables were analyzed using the chi-squared test or Fisher's exact test, as appropriate. Continuous variables were analyzed using the unpaired t test or Mann-Whitney U test, as appropriate. A P value of <0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 23.0 (IBM, Armonk, NY, USA).
Results
Evaluation of monophasic and polyphasic waves
Table 1 shows the results of evaluation of the waveforms in terms of the type of LSR. The polyphasic wave consisted of long duration waves that appeared with both temporal and marginal mandibular branch stimulation. In this study, the initial LSR morphology showed no mixing of polyphasic and monophasic waves despite several stimulations during decision-making regarding each patient's initial wave.
Table 1.
Evaluation results of lateral spread response
| Evaluation of each waveform | Polyphasic wave | Monophasic wave | P value |
|---|---|---|---|
| Temporal branch stimulation, n | 5 | 36 | |
| Number of spikes (mean ± SD), n | 5.4 ± 3.2 | 1.4 ± 0.5 | 0.00 |
| Duration (mean ± SD), ms | 30 ± 10.6 | 20.6 ± 6.7 | 0.00 |
| Maximum amplitude (mean ± SD), μV | 124.4 ± 72.1 | 109.9 ± 83.7 | 0.36 |
| Marginal mandibular branch stimulation, n | 17 | 24 | |
| Number of spikes (mean ± SD), n | 4.3 ± 2.8 | 1.6 ± 0.5 | 0.00 |
| Duration (mean ± SD), ms | 29.4 ± 7.9 | 22.5 ± 6.6 | 0.00 |
| Maximum amplitude (mean ± SD), μV | 58.1 ± 37.7 | 86.1 ± 92.0 | 0.12 |
Polyphasic waves, consisting of waves with a long duration, appeared with both temporal and marginal mandibular branch stimulation. SD, standard deviation
Clinical information
We analyzed the data of 41 of 152 consecutive patients who underwent MVD for HFS at our hospital during the study period, after excluding 66 patients with a past history of botulinum neurotoxin injection, 2 patients with prior MVD surgery, 25 patients with an unmeasurable initial LSR or in whom all the waveform amplitudes were less than 10 μV, 3 patients with preoperative facial weakness, and 18 patients who were lost to follow-up of CMAP. None of the patients had a history of Bell's palsy, trauma, or other surgical treatment around their FN area, but three cases had preoperative facial weakness. Table 2 shows the general characteristics of the subjects stratified according to the pattern of the initial LSR. Baseline data did not differ between the two groups, except for the percentage of cases in which the anterior inferior cerebellar artery (AICA) was the offending vessel. None of the patients had the complication of facial palsy at the time of follow-up.
Table 2.
Baseline characteristics of the study groups
| Baseline characteristics | Polyphasic group (n = 19) |
Monophasic group (n = 22) |
P value |
|---|---|---|---|
| Age (mean ± SD), years | 53.3 ± 12.5 | 51.3 ± 13.7 | 0.31 |
| Male, n (%) | 5 (26.3) | 13 (59.1) | 0.07 |
| Side (left), n (%) | 12 (63.2) | 15 (68.2) | 0.99 |
| Symptom duration (mean ± SD), months | 57.1 ± 49.0 | 60.3 ± 72.6 | 0.43 |
| Risk factors, n (%) | |||
| Hypertension | 5 (26.3) | 2 (9.1) | 0.22 |
| Diabetes mellitus | 2 (10.5) | 0 (0.0) | 0.21 |
| Dyslipidemia | 2 (10.5) | 3 (13.6) | 1 |
| Offending vessels, n (%) | |||
| AICA | 17 (89.5) | 12 (54.5) | 0.02 |
| PICA | 5 (26.3) | 10 (45.5) | 0.35 |
| VA | 3 (15.8) | 9 (40.9) | 0.1 |
| Complex | 6 (31.6) | 7 (31.8) | 0.75 |
Baseline data did not differ between the two groups, except for the percentage of cases in which the AICA was the offending vessel. SD, standard deviation; AICA, anterior inferior cerebellar artery; PICA, posterior inferior cerebellar artery; VA, vertebral artery
Primary outcome measures
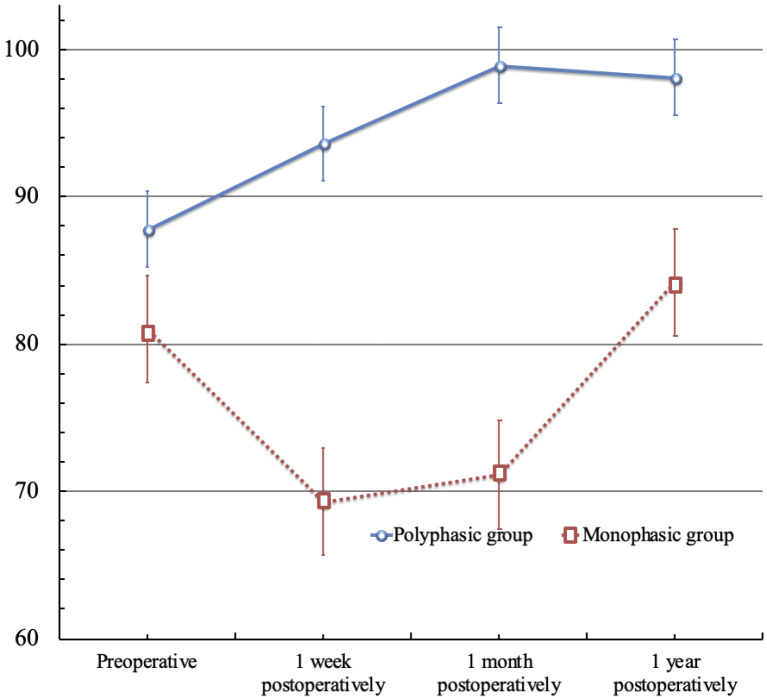
Figure 2 shows the progress of CMAP. In the polyphasic group, the amplitude ratio of the CMAP (amplitude of CMAP on the affected side/amplitude of CMAP on the unaffected side) tended to gradually improve with time, whereas in the monophasic group, the amplitude ratio of the CMAP decreased on the seventh postoperative day, followed by its gradual improvement (polyphasic group/monophasic group; preoperative, 87.8 ± 37.9/81.0 ± 31.6, P = 0.54, 1 week postoperative, 93.6 ± 40.5/69.3 ± 28.5, P = 0.03, 1 month postoperative, 98.9 ± 57.9/71.1 ± 34.0, P = 0.06, 1 year postoperative, 98.1 ± 29.2/84.2 ± 30.1, P = 0.14).
Fig. 2.
Progress of compound motor action potential.
Figure 2 shows the progress of CMAP. In the polyphasic group, the amplitude ratio of the CMAP (amplitude of CMAP of the affected side/amplitude of CMAP of the unaffected side) tended to improve gradually with time, whereas in the monophasic group, the amplitude ratio of the CMAP decreased on the seventh postoperative day, followed by gradual improvement (polyphasic group/monophasic group; preoperative, 87.8±37.9/81.0±31.6, P=0.54, 1 week postoperative, 93.6±40.5/69.3±28.5, P=0.03, 1 month postoperative, 98.9±57.9/71.1±34.0, P=0.06, 1 year postoperative, 98.9±57.9/71.1±34.0, P=0.06, 1 year postoperative, 98.1±29.2/84.2±30.1, P=0.14).
Other parameters
There were significantly higher rates of residual postoperative HFS in the polyphasic group at 1 week and 1 month after the surgery (P < 0.05, respectively), as assessed using Yates' chi-squared test and Fisher's exact test (Table 3). Conversely, the rate of residual postoperative HFS at 1 year and final follow-up after surgery did not differ between the two groups (P = 0.46).
Table 3.
Clinical outcomes of the study groups
| Clinical outcomes | Polyphasic group (n = 19) |
Monophasic group (n = 22) |
P value |
|---|---|---|---|
| Follow-up period (mean ± SD), months | 43.9 ± 24.5 | 50.6 ± 29.5 | 0.22 |
| Residual spasm, n (%) | |||
| Within 1 week | 10 (52.6) | 3 (13.6) | 0.02 |
| Within 1 month | 8 (42.1) | 1 (4.5) | 0.01 |
| Within 1 year | 1 (5.3) | 0 (0.0) | 0.46 |
| Final follow-up | 1 (5.3) | 0 (0.0) | 0.46 |
| Intraoperative AMR disappearance, n (%) | 17 (89.5) | 15 (68.2) | 0.14 |
| Complications | |||
| Subdural hemorrhage | 0 (0.0) | 1 (4.5) | 1 |
| Hoarseness | 1 (5.3) | 0 (0.0) | 0.46 |
| Dysphagia | 1 (5.3) | 1 (4.5) | 1 |
There were significantly higher rates of residual postoperative hemifacial spasm in the polyphasic group at both 1 week and 1 month after the surgery (P < 0.05, respectively), as assessed using Yates’ chi-squared test and Fisher’s exact test.
Intraoperative LSR remained in 2 cases in the polyphasic group and 7 cases in the monophasic group, with no difference between the 2 groups. Of these 9 patients, 4 had delayed relief of HFS, and the frequency of delayed relief was 44.4%.
There were no differences in permanent complications rate between the two groups.
Discussion
Although MVD is a reliable treatment for HFS, the exact reasons for surgical failure remain unclear. In particular, delayed postoperative cure of persistent HFS is one of the most challenging issues. In previous reports, intraoperative LSR, the pattern of neurovascular compression, good clinical outcomes at 3 months after MVD, and intraoperative evidence of the severity of the REZ indentation were proposed as prognostic factors for better outcomes.17,26,27) The percentage of delayed cures in the polyphasic group in the present study (52.6% at 1 week after surgery and 42.1% at 1 month after surgery) was higher than those reported in previous reports (10%-40%).13-20) Our results suggest that the initial morphology of the LSR might be an important factor for estimating a delayed cure after MVD. To our knowledge, only one previous paper has evaluated the correlation between intraoperative morphology of the LSR and cure rates of HFS after MVD, although they did not examine the correlation between the morphology of the LSR and delayed cure.28)
Mechanism of polyphasic wave formation in LSRs
Previous studies have proposed two possible sites of origin for the lateral spread of impulses responsible for the LSR seen in HFS: (1) coactivation of many abnormal hyperexcitable motoneurons following antidromic invasion or reflexive activation of the facial nucleus22,29) or (2) ephaptic transmission at the vascular compression site along the intracranial extra-axial segment of the FN.30-34) In addition to the above possible origins for lateral spread of the impulse, the electrical shocks intended for the FN could simultaneously activate cutaneous sensory fibers, inducing a blink reflex.35,36) Recent studies suggest the role of cutaneous afferent volleys via the trigeminal nerve, which enhances the reflexive excitability of the FN motoneurons. Elicitation of an LSR after subthreshold stimulation of the FN also suggests mediation of an aberrant impulse by trigeminal inputs.36) Therefore, the LSR waveform is considered to be the sum of several components of ephaptic transmission at the vascular compression site or coactivation of many abnormal hyperexcitable motoneurons and a waveform evoked by the blink reflex.
Two factors might cause the LSR waveform to be polyphasic. One is the degree of demyelination at the vascular compression site. Several papers have shown that demyelination of the FN is one of the mechanisms of HFS37,38) and that the morphology of the wave in polyphasic-type LSRs is very similar to the demyelinating wave of temporal dispersion seen in electrophysiological evaluation in patients with demyelinating polyneuropathy, such as chronic inflammatory demyelinating polyneuropathy, or Guillain-Barré syndrome.39-43) When depolarization reaches a critical level due to contralateral stimulation, the voltage-gated Na channel opens, leading to generation of an action potential. The action potential is constant regardless of the type and size of the stimulus during the generation process, thus obeying the all-or-none law.44-47) Therefore, the LSR waveform is considered to be a change that strongly reflects the effect of demyelination after ephaptic transmission. Our results suggest that the presence of the polyphasic LSR is associated with the degree of demyelination of the temporal and mandibular branches of the FN at the vascular compression site. In cases that showed both monophasic and polyphasic waveforms, the degree of demyelination differed between the temporal and mandibular branches, and multilayered waveforms were only seen when the signal was transmitted to the side with the stronger demyelination.
The other factor that might be responsible for the polyphasic waveform is involvement of the blink reflex. Blink reflexes are frequently present in patients with FHS.48-52) The blink reflex elicited by electrical stimulation of the supraorbital nerve has two orbicularis oculi contraction components: one with a latency of 10 to 12 ms (R1), and the other with a latency of 30-40 ms (R2).53,54) While the blink reflex seldom involves muscles other than the orbicularis oculi in normal subjects, it usually involves other muscles in patients with HFS.55,56) This is consistent with our results, which showed significantly more polyphasic waves during orbicularis oculi recordings following stimulation of the mandibular branch of the FN. Stimulation by the electrode would activate peripheral nerve branches of the trigeminal nerve or skin sensory receptors, and thereby, could elicit trigeminal facial reflexes. The impulse then travels afferently to the FN nucleus. We believe that the threshold for signal propagation from the FN nucleus might be lower in cases with hyperexcitation of the FN nucleus, leading to a greater likelihood of induction of blink reflexes, although further case accumulation and investigation are needed.
Causative vessel and LSR waveform
In this study, the AICA was found to be the offending vessel significantly more often in the polyphasic group than in the monophasic group. Previous studies have also described that the AICA was the vessel most frequently responsible for compression at the site of the FN REZ.57,58)
The FN REZ is commonly defined as the proximal segment of the nerve, from the facial root exit point to the transition zone.59-61) With oligodendrocyte-derived myelin, the REZ is structurally weaker and more vulnerable to the influence of vascular compression.62,63) In addition, the REZ of the healthy FN is ensheathed by only the arachnoid membrane and lacks interfascicular connective tissue that usually separates the fibers and epineurium.62,64) These anatomical characteristics might make the REZ slightly more vulnerable to injury by vascular compression.65) This supports the observation of the greater frequency of the AICA as the offending vessel causing compression of the anatomically fragile REZ in the polyphasic group, in which demyelination was observed to be more strongly involved.
Considerations related to CMAP progression
The CMAP is a summation of the action potentials of muscle fibers in a given muscle group and is an indication of the number of functioning muscle fibers. To our knowledge, few studies have objectively investigated motor function of the FN in HFS patients and assessed the long-term influence of surgery on the facial motor nerve. Asayama et al. reported the first study to identify the characteristics and clinical outcomes of preoperative and postoperative facial motor weakness in HFS patients, which examined the long-term sequential electrophysiological changes in FN motor function pre- and post-MVD in patients with HFS.25) They showed that CMAP amplitude values were significantly lower in patients with preoperative facial motor weakness, who were considered to have strong axonal degeneration, than in other patients.
In the polyphasic group in this study, early recovery of the CMAP was observed starting on postoperative day 7, with a small decrease in amplitude, resembling the changes seen in segmental demyelination with little damage to the stimulating site of the peripheral nerve. In the monophasic group, there was a marked decrease in amplitude, which was considered a strong finding of axonal degeneration. Previous reports have described the pathology of early Wallerian degeneration in the stroke area, which is seen from a few days to a week after the onset.66-69) We previously identified imaging changes suggestive of early Wallerian degeneration at the first week after MVD for trigeminal neuralgia, which might be evidence of the axonal degeneration associated with intraoperative intervention.70) This is consistent with the timing of the decline in CMAP, i.e., at 1 week postoperatively in the monophasic group. The course of CMAP in this study, in which cases without Bell's palsy or Botox treatment and without pre- or postoperative facial palsy were selected, allowed us to confirm the obliterative nerve changes caused by intracranial FN injury. It is possible that cases with more severe axonal damage in the monophasic group developed more pronounced inflammatory changes secondary to mechanical stimulation during MVD, which might have resulted in the progression of Wallerian degeneration.
Correlation between demyelination of the facial nerve and delayed cure from HFS after MVD
Based on the current understanding of the pathophysiological mechanisms underlying HFS, vascular compression of the FN seems to play a crucial role in HFS.3) Previous studies have suggested that irritation of the FN due to its close contact with a blood vessel promotes hyperactivity and hyperexcitability of the FN nucleus.33) As in previous reports,20) there was no correlation between the intraoperative LSR disappearance rate and outcomes at 1 year in this study, suggesting that hyperexcitability of the facial nucleus might normalize progressively over several months or even years after MVD. This process of normalizing the hyperexcitability of the nucleus is thought to manifest as delayed cure of HFS.71,72) The likelihood of triggering blink reflexes might indicate hyperexcitability of the facial nucleus, and evaluation of polyphasic waveforms can provide real-time visualization of hyperexcitability of the nucleus. In addition to these theories, we suggest that demyelination of the FN at the vascular compression site might enhance facial muscle excitability, leading to spatial dispersion from the facial nucleus to lower threshold motor neurons of the FN. Alternatively, due to the state of strong axonal degeneration in the monophasic group, impulses from the FN nucleus were less likely to propagate to the periphery, causing the facial spasm to disappear immediately after surgery.
This study has some limitations. First, since this was a retrospective study of a single center with a small sample size, further studies with a larger sample size are required to confirm our results. Second, we evaluated residual postoperative HFS 1 week, 1 month, and 1 year and final follow-up after the surgery. We need to accumulate more detailed follow-up data to determine the appropriate timing for evaluation of delayed cure after MVD.
Conclusion
There is a significant correlation between delayed relief after MVD and polyphasic morphology of the initial LSR in patients with HFS. In the polyphasic group, CMAP recovered earlier and was less amplitudinally reduced, suggesting similar changes as seen in segmental demyelination, with less damage to peripheral nerves.
Funding Sources
The author received no funding for this work.
Conflicts of Interest Disclosure
No company had influence on or knowledge of the results of this study.
References
- 1). Barker FG, Jannetta PJ, Bissonette DJ, Shields PT, Larkins MV, Jho HD: Microvascular decompression for hemifacial spasm. J Neurosurg 82: 201-210, 1995 [DOI] [PubMed] [Google Scholar]
- 2). Auger RG: Hemifacial spasm: clinical and electrophysiologic observations. Neurology 29: 1261-1272, 1979 [DOI] [PubMed] [Google Scholar]
- 3). Jannetta PJ, Abbasy M, Maroon JC, Ramos FM, Albin MS: Etiology and definitive microsurgical treatment of hemifacial spasm. Operative techniques and results in 47 patients. J Neurosurg 47: 321-328, 1977 [DOI] [PubMed] [Google Scholar]
- 4). Jannetta PJ: Hemifacial spasm: treatment by posterior fossa surgery. J Neurol Neurosurg Psychiatry 46: 465-466, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Møller AR: Interaction between the blink reflex and the abnormal muscle response in patients with hemifacial spasm: results of intraoperative recordings. J Neurol Sci 101: 114-123, 1991 [DOI] [PubMed] [Google Scholar]
- 6). Møller AR: The cranial nerve vascular compression syndrome: I. A review of treatment. Acta Neurochir 113: 18-23, 1991 [DOI] [PubMed] [Google Scholar]
- 7). Huang CI, Chen IH, Lee LS: Microvascular decompression for hemifacial spasm: analyses of operative findings and results in 310 patients. Neurosurgery 30: 53-56; discussion 56, 1992 [DOI] [PubMed] [Google Scholar]
- 8). Illingworth RD, Porter DG, Jakubowski J: Hemifacial spasm: a prospective long-term follow up of 83 cases treated by microvascular decompression at two neurosurgical centres in the United Kingdom. J Neurol Neurosurg Psychiatry 60: 72-77, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Samii M, Günther T, Iaconetta G, Muehling M, Vorkapic P, Samii A: Microvascular decompression to treat hemifacial spasm: long-term results for a consecutive series of 143 patients. Neurosurgery 50: 712-718; discussion 718, 2002 [DOI] [PubMed] [Google Scholar]
- 10). Huh R, Han IB, Moon JY, Chang JW, Chung SS: Microvascular decompression for hemifacial spasm: analyses of operative complications in 1582 consecutive patients. Surg Neurol 69: 153-157; discussion 157, 2008 [DOI] [PubMed] [Google Scholar]
- 11). Shibahashi K, Morita A, Kimura T: Surgical results of microvascular decompression procedures and patient's postoperative quality of life: review of 139 cases. Neurol Med Chir (Tokyo) 53: 360-364, 2013 [DOI] [PubMed] [Google Scholar]
- 12). Mizobuchi Y, Muramatsu K, Ohtani M, et al. : The current status of microvascular decompression for the treatment of hemifacial spasm in Japan: an analysis of 2907 patients using the Japanese diagnosis procedure combination database. Neurol Med Chir (Tokyo) 57: 184-190, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Hatayama T, Kono T, Harada Y, et al. : Indications and timings of re-operation for residual or recurrent hemifacial spasm after microvascular decompression: personal experience and literature review. Neurol Med Chir (Tokyo) 55: 663-668, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Hatem J, Sindou M, Vial C: Intraoperative monitoring of facial EMG responses during microvascular decompression for hemifacial spasm. Prognostic value for long-term outcome: a study in a 33-patient series. Br J Neurosurg 15: 496-499, 2001 [DOI] [PubMed] [Google Scholar]
- 15). Ishikawa M, Nakanishi T, Takamiya Y, Namiki J: Delayed resolution of residual hemifacial spasm after microvascular decompression operations. Neurosurgery 49: 847-854; discussion 854, 2001 [DOI] [PubMed] [Google Scholar]
- 16). Kim CH, Kong DS, Lee JA, Park K: The potential value of the disappearance of the lateral spread response during microvascular decompression for predicting the clinical outcome of hemifacial spasms: a prospective study. Neurosurgery 67: 1581-1588, 2010 [DOI] [PubMed] [Google Scholar]
- 17). Kong DS, Park K, Shin BG, Lee JA, Eum DO: Prognostic value of the lateral spread response for intraoperative electromyography monitoring of the facial musculature during microvascular decompression for hemifacial spasm. J Neurosurg 106: 384-387, 2007 [DOI] [PubMed] [Google Scholar]
- 18). Shin JC, Chung UH, Kim YC, Park CI: Prospective study of microvascular decompression in hemifacial spasm. Neurosurgery 40: 730-734; discussion 734, 1997 [DOI] [PubMed] [Google Scholar]
- 19). Terasaka S, Asaoka K, Yamaguchi S, Kobayashi H, Motegi H, Houkin K: A significant correlation between delayed cure after microvascular decompression and positive response to preoperative anticonvulsant therapy in patients with hemifacial spasm. Neurosurg Rev 39: 607-613, 2016 [DOI] [PubMed] [Google Scholar]
- 20). Tobishima H, Hatayama T, Ohkuma H: Relation between the persistence of an abnormal muscle response and the long-term clinical course after microvascular decompression for hemifacial spasm. Neurol Med Chir (Tokyo) 54: 474-482, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Sato Y, Shimizu K, Iizuka K, Irie R, Matsumoto M, Mizutani T: Factors related to the delayed cure of hemifacial spasm after microvascular decompression: an analysis of 175 consecutive patients. J Neurol Surg B Skull Base 83: 548-553, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Møller AR: Hemifacial spasm: ephaptic transmission or hyperexcitability of the facial motor nucleus? Exp Neurol 98: 110-119, 1987 [DOI] [PubMed] [Google Scholar]
- 23). Møller AR: The cranial nerve vascular compression syndrome: II. A review of pathophysiology. Acta Neurochir 113: 24-30, 1991 [DOI] [PubMed] [Google Scholar]
- 24). Amano Y, Asayama B, Noro S, et al. : Significant correlation between delayed relief after microvascular decompression and morphology of the abnormal muscle response in patients with hemifacial spasm. Neurol Med Chir (Tokyo) 62: 513-520, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Asayama B, Noro S, Abe T, Seo Y, Honjo K, Nakamura H: Sequential change of facial nerve motor function after microvascular decompression for hemifacial spasm: an electrophysiological study. Neurol Med Chir (Tokyo) 61: 461-467, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Kim HR, Rhee DJ, Kong DS, Park K: Prognostic factors of hemifacial spasm after microvascular decompression. J Korean Neurosurg Soc 45: 336-340, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Sekula RF, Bhatia S, Frederickson AM, et al. : Utility of intraoperative electromyography in microvascular decompression for hemifacial spasm: a meta-analysis. Neurosurg Focus 27: E10, 2009 [DOI] [PubMed] [Google Scholar]
- 28). Hirono S, Yamakami I, Sato M, et al. : Continuous intraoperative monitoring of abnormal muscle response in microvascular decompression for hemifacial spasm; a real-time navigator for complete relief. Neurosurg Rev 37: 311-319; discussion 319, 2014 [DOI] [PubMed] [Google Scholar]
- 29). Møller AR, Jannetta PJ: Hemifacial spasm: results of electrophysiologic recording during microvascular decompression operations. Neurology 35: 969-974, 1985 [DOI] [PubMed] [Google Scholar]
- 30). Nielsen VK: Pathophysiology of hemifacial spasm: I. Ephaptic transmission and ectopic excitation. Neurology 34: 418-426, 1984 [DOI] [PubMed] [Google Scholar]
- 31). Nielsen VK: Pathophysiology of hemifacial spasm: II. Lateral spread of the supraorbital nerve reflex. Neurology 34: 427-431, 1984 [DOI] [PubMed] [Google Scholar]
- 32). Nielsen VK, Jannetta PJ: Pathophysiology of hemifacial spasm: III. Effects of facial nerve decompression. Neurology 34: 891-897, 1984 [DOI] [PubMed] [Google Scholar]
- 33). Nielsen VK: Electrophysiology of the facial nerve in hemifacial spasm: ectopic/ephaptic excitation. Muscle Nerve 8: 545-555, 1985 [DOI] [PubMed] [Google Scholar]
- 34). Kameyama S, Masuda H, Shirozu H, Ito Y, Sonoda M, Kimura J: Ephaptic transmission is the origin of the abnormal muscle response seen in hemifacial spasm. Clin Neurophysiol 127: 2240-2245, 2016 [DOI] [PubMed] [Google Scholar]
- 35). Ogawara K, Kuwabara S, Kamitsukasa I, Mizobuchi K, Misawa S, Hattori T: Trigeminal afferent input alters the excitability of facial motoneurons in hemifacial spasm. Neurology 62: 1749-1752, 2004 [DOI] [PubMed] [Google Scholar]
- 36). Misawa S, Kuwabara S, Ogawara K, Hattori T: Abnormal muscle responses in hemifacial spasm: F waves or trigeminal reflexes? J Neurol Neurosurg Psychiatry 77: 216-218, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). Kuroki A, Møller AR: Facial nerve demyelination and vascular compression are both needed to induce facial hyperactivity: a study in rats. Acta Neurochir 126: 149-157, 1994 [DOI] [PubMed] [Google Scholar]
- 38). Micheli F, Scorticati MC, Gatto E, Cersosimo G, Adi J: Familial hemifacial spasm. Mov Disord 9: 330-332, 1994 [DOI] [PubMed] [Google Scholar]
- 39). Griffin JW, Li CY, Ho TW, et al. : Guillain-Barré syndrome in northern China. The spectrum of neuropathological changes in clinically defined cases. Brain 118: 577-595, 1995 [DOI] [PubMed] [Google Scholar]
- 40). Bouchard C, Lacroix C, Planté V, et al. : Clinicopathologic findings and prognosis of chronic inflammatory demyelinating polyneuropathy. Neurology 52: 498-503, 1999 [DOI] [PubMed] [Google Scholar]
- 41). Kuwabara S, Ogawara K, Misawa S, Mori M, Hattori T: Distribution patterns of demyelination correlate with clinical profiles in chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry 72: 37-42, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42). Kuwabara S, Yuki N: Axonal Guillain-Barré syndrome: concepts and controversies. Lancet Neurol 12: 1180-1188, 2013 [DOI] [PubMed] [Google Scholar]
- 43). Uncini A, Kuwabara S: The electrodiagnosis of Guillain-Barré syndrome subtypes: where do we stand? Clin Neurophysiol 129: 2586-2593, 2018 [DOI] [PubMed] [Google Scholar]
- 44). Bostock H, Sears TA: The internodal axon membrane: electrical excitability and continuous conduction in segmental demyelination. J Physiol 280: 273-301, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45). Bostock H, Sherratt RM, Sears TA: Overcoming conduction failure in demyelinated nerve fibres by prolonging action potentials. Nature 274: 385-387, 1978 [DOI] [PubMed] [Google Scholar]
- 46). Burke D, Kiernan MC, Bostock H: Excitability of human axons. Clin Neurophysiol 112: 1575-1585, 2001 [DOI] [PubMed] [Google Scholar]
- 47). Kocsis JD, Waxman SG: Absence of potassium conductance in central myelinated axons. Nature 287: 348-349, 1980 [DOI] [PubMed] [Google Scholar]
- 48). Møller AR, Jannetta PJ: Blink reflex in patients with hemifacial spasm. Observations during microvascular decompression operations. J Neurol Sci 72: 171-182, 1986 [DOI] [PubMed] [Google Scholar]
- 49). Kim P, Fukushima T: Observations on synkinesis in patients with hemifacial spasm. Effect of microvascular decompression and etiological considerations. J Neurosurg 60: 821-827, 1984 [DOI] [PubMed] [Google Scholar]
- 50). Ishikawa M, Ohira T, Namiki J, Ajimi Y, Takase M, Toya S: Abnormal muscle response (lateral spread) and F-wave in patients with hemifacial spasm. J Neurol Sci 137: 109-116, 1996 [DOI] [PubMed] [Google Scholar]
- 51). Ishikawa M, Ohira T, Namiki J, et al. : Electrophysiological investigation of hemifacial spasm after microvascular decompression: F waves of the facial muscles, blink reflexes, and abnormal muscle responses. J Neurosurg 86: 654-661, 1997 [DOI] [PubMed] [Google Scholar]
- 52). Møller AR, Sen CN: Recordings from the facial nucleus in the rat: signs of abnormal facial muscle response. Exp Brain Res 81: 18-24, 1990 [DOI] [PubMed] [Google Scholar]
- 53). Kimura J: Clinical uses of the electrically elicited blink reflex. Adv Neurol 39: 773-786, 1983 [PubMed] [Google Scholar]
- 54). Fine EJ, Sentz L, Soria E: The history of the blink reflex. Neurology 42: 450-454, 1992 [DOI] [PubMed] [Google Scholar]
- 55). Auger RG, Piepgras DG, Laws ER, Miller RH: Microvascular decompression of the facial nerve for hemifacial spasm: clinical and electrophysiologic observations. Neurology 31: 346-350, 1981 [DOI] [PubMed] [Google Scholar]
- 56). Ishikawa M, Shinoda S, Watanabe E: Hyperexcitability of the facial motor nucleus in patients with hemifacial spasm: analysis of F waves in facial muscles. Jpn J Neurosurg (Tokyo) 19: 50-56, 2010 [Google Scholar]
- 57). Jiang C, Liang W, Wang J, et al. : Microvascular decompression for hemifacial spasm associated with distinct offending vessels: a retrospective clinical study. Clin Neurol Neurosurg 194: 105876, 2020 [DOI] [PubMed] [Google Scholar]
- 58). Naraghi R, Tanrikulu L, Troescher-Weber R, et al. : Classification of neurovascular compression in typical hemifacial spasm: three-dimensional visualization of the facial and the vestibulocochlear nerves. J Neurosurg 107: 1154-1163, 2007 [DOI] [PubMed] [Google Scholar]
- 59). Campos-Benitez M, Kaufmann AM: Neurovascular compression findings in hemifacial spasm. J Neurosurg 109: 416-420, 2008 [DOI] [PubMed] [Google Scholar]
- 60). Eidelman BH, Nielsen VK, Møller M, Jannetta PJ: Vascular compression, hemifacial spasm, and multiple cranial neuropathy. Neurology 35: 712-716, 1985 [DOI] [PubMed] [Google Scholar]
- 61). Guclu B, Sindou M, Meyronet D, Streichenberger N, Simon E, Mertens P: Cranial nerve vascular compression syndromes of the trigeminal, facial and vago-glossopharyngeal nerves: comparative anatomical study of the central myelin portion and transitional zone; correlations with incidences of corresponding hyperactive dysfunctional syndromes. Acta Neurochir 153: 2365-2375, 2011 [DOI] [PubMed] [Google Scholar]
- 62). De Ridder D, Møller A, Verlooy J, Cornelissen M, De Ridder LD: Is the root entry/exit zone important in microvascular compression syndromes? Neurosurgery 51: 427-433; discussion 433, 2002 [DOI] [PubMed] [Google Scholar]
- 63). Yee GT, Yoo CJ, Han SR, Choi CY: Microanatomy and histological features of central myelin in the root exit zone of facial nerve. J Korean Neurosurg Soc 55: 244-247, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64). Lu AY, Yeung JT, Gerrard JL, Michaelides EM, Sekula RF Jr, Bulsara KR: Hemifacial spasm and neurovascular compression. ScientificWorldJournal 2014: 349319, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65). Ziyal IM, Özgen T: Microanatomy of the central myelin-peripheral myelin transition zone of the trigeminal nerve. Neurosurgery 60: E582; author reply E582, 2007 [DOI] [PubMed] [Google Scholar]
- 66). DeVetten GD, Coutts SB, Hill MD, et al. : Acute corticospinal tract Wallerian degeneration is associated with stroke outcome. Stroke 41: 751-756, 2010 [DOI] [PubMed] [Google Scholar]
- 67). Uchino A, Sawada A, Takase Y, Egashira R, Kudo S: Transient detection of early wallerian degeneration on diffusion-weighted MRI after an acute cerebrovascular accident. Neuroradiology 46: 183-188, 2004 [DOI] [PubMed] [Google Scholar]
- 68). Yamada K, Kizu O, Ito H, et al. : Wallerian degeneration of the inferior cerebellar peduncle depicted by diffusion weighted imaging. J Neurol Neurosurg Psychiatry 74: 977-978, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69). Mazumdar A, Mukherjee P, Miller JH, Malde H, McKinstry RC: Diffusion-weighted imaging of acute corticospinal tract injury preceding Wallerian degeneration in the maturing human brain. AJNR Am J Neuroradiol 24: 1057-1066, 2003 [PMC free article] [PubMed] [Google Scholar]
- 70). Amano Y, Asayama B, Noro S, et al. : Objectively-captured changes in trigeminal fibers before and after microvascular decompression using 3D T2-SPACE MRI might relate to eventual residual symptoms. Neurol Med Chir (Tokyo) 63: 400-408, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71). Esteban A, Molina-Negro P: Primary hemifacial spasm: a neurophysiological study. J Neurol Neurosurg Psychiatry 49: 58-63, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72). Valls-Sole J, Tolosa ES: Blink reflex excitability cycle in hemifacial spasm. Neurology 39: 1061-1066, 1989 [DOI] [PubMed] [Google Scholar]