Abstract
A 68-year-old woman with follicular lymphoma was treated with chemotherapy, including the anti-CD20 monoclonal antibody obinutuzumab, and achieved remission. A month after the administration of obinutuzumab, the patient contracted coronavirus disease 2019 (COVID-19), and various antiviral drugs were administered. However, the infection had not been eliminated. One year and three months after chemotherapy, peripheral B cell recovery was achieved, and the infection had resolved. This report provides insight into the course of COVID-19 in patients with impaired humoral immunity.
Keywords: Anti-CD20 antibody, B-cell depletion therapy, Follicular lymphoma, Obinutuzumab, Persistent SARS-CoV-2 infection
1. Introduction
Patients with hematological malignancies are vulnerable to coronavirus disease 2019 (COVID-19) because of the immunosuppression caused by underlying diseases and drugs used to treat the malignancy [1]. In particular, the prognosis may be worse in patients who receive obinutuzumab, which has a higher tumoricidal effect than conventional rituximab [2,3]. The course and treatment of COVID-19 in patients with impaired humoral immunity remain unclear.
We report the case of a patient infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with impaired humoral immunity caused by B-cell-depletion therapy. After approximately 1 year and 3 months after the last administration of obinutuzumab, B cells were restored, and the SARS-CoV-2 infection had resolved.
2. Case report
A 68-year-old woman diagnosed with follicular lymphoma (FL) underwent six cycles of bendamustine and obinutuzumab (GB) induction chemotherapy from March to August 2021, followed by eight cycles of obinutuzumab maintenance therapy until January 2023. The lymphoma was in complete remission by November 2022 based on positron emission tomography/computed tomography. During the GB induction phase, the patient received two doses of anti-SARS-CoV-2 mRNA vaccination (Pfizer-BioNTech) on July 24 and August 14, 2021. During the obinutuzumab maintenance phase, she received a third vaccination on March 17, 2022.
At the beginning of February 2023, she visited a local doctor because of high fever and cough. She was diagnosed with COVID-19 via a SARS-CoV-2 qualitative antigen test and was treated with molnupiravir. Her symptoms became milder but a high fever recurred in mid-February. The patient presented to our hospital's emergency department in early March with a high fever and cough. Real-time polymerase chain reaction (RT-PCR) testing of nasopharyngeal swab (NPS) specimens yielded positive results for SARS-CoV-2. Based on the epidemic situation in the residential areas, the virus was identified as an Omicron variant.
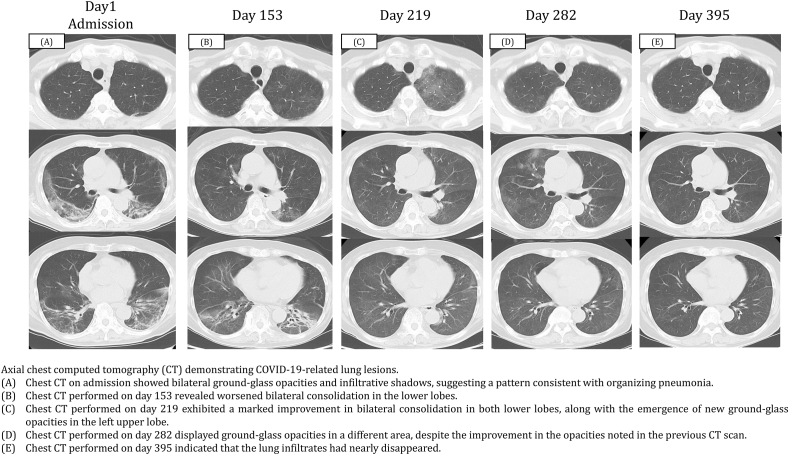
The patent's body temperature was 37.9 °C, blood pressure was 131/82 mmHg, heart rate was 70 beats/min, and respiratory rate was 12 breaths/min with percutaneous oxygen saturation of 96 % on room air. The WBC count was 2400/μL with 79.0 % neutrophils, 10.5 % lymphocytes, 0.5 % basophils, and 10.0 % monocytes. The levels of C-reactive protein (CRP), lactate dehydrogenase, surfactant protein-D, Krebs von den Lungen-6, and soluble interleukin-2 receptor were 10.3 mg/dL (normal range: 0–0.3 mg/dL), 378 U/L (normal range: 124–222 U/L), 38.5 ng/mL (normal range: <110 ng/mL), 443 U/mL (normal range: <500 U/mL), and 1111 U/mL (normal range: 127–582 U/mL), respectively. Chest computed tomography (CT) revealed subpleural ground-glass opacities and infiltrative shadows in both lungs (Fig. 1A).
Fig. 1.
Axial chest computed tomography (CT) demonstrating COVID-19-related lung lesions.
(A) Chest CT on admission showed bilateral ground-glass opacities and infiltrative shadows, suggesting a pattern consistent with organizing pneumonia. (B) Chest CT performed on day 153 revealed worsened bilateral consolidation in the lower lobes. (C) Chest CT performed on day 219 exhibited a marked improvement in bilateral consolidation in both lower lobes, along with the emergence of new ground-glass opacities in the left upper lobe. (D) Chest CT performed on day 282 displayed ground-glass opacities in a different area, despite the improvement in the opacities noted in the previous CT scan. (E) Chest CT performed on day 395 indicated that the lung infiltrates had nearly disappeared.
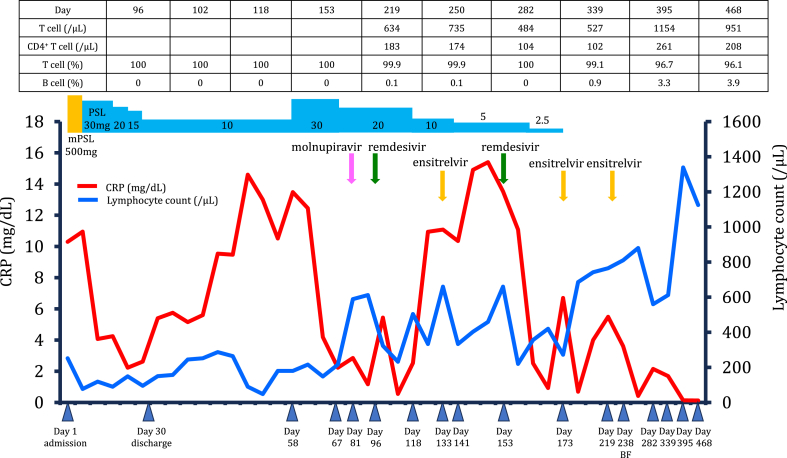
Approximately 1 month after the onset of COVID-19, she was diagnosed with community-acquired pneumonia and interstitial lung disease after COVID-19 and hospitalized (Fig. 2). Sulbactam/ampicillin was administered at a dosage of 9 g for 7 days, and methylprednisolone (500 mg/day) was administered intravenously for 3 days. This was followed by the oral administration of prednisolone (PSL) at 30 mg/day. Her respiratory symptoms improved, and the PSL dose was tapered weekly. The patient was discharged on day 30 and was prescribed 10 mg of PSL administered orally daily but returned to the hospital on day 58 after experiencing a high fever lasting several days. The CRP level remained elevated (13.58 mg/dL). Chest CT revealed worsening infiltrative shadows and ground-glass opacities in both lungs, and the PSL dose was increased to 30 mg/day.
Fig. 2.
The clinical course of over 15 months of persistent active SARS-CoV-2 infection in a patient receiving anti-CD20 therapy for follicular lymphoma.
Additional blood tests were performed, considering the possibility of immunosuppression caused by anti-CD20 monoclonal antibodies. Anti-SARS-CoV-2 nucleocapsid-protein and spike-protein antibody tests yielded negative results. Serum immunoglobulin (Ig) A (58.0 mg/dL), IgG (547.0 mg/dL), and IgM (13.0 mg/dL) levels were low, and the peripheral blood B-cell frequency was 0 %. Antigen detection and RT-PCR NPS tests were positive, with a cycle threshold (Ct) value of 24.75 (tested against the N2 gene). A diagnosis of pneumonia caused by persistent SARS-CoV-2 infection was confirmed on day 67.
We speculated that continued PSL administration has an additional effect on impaired immunity, resulting in fever and persistent CRP positivity caused by prolonged SARS-CoV-2 infection. Therefore, we decided to gradually reduce the dosage of PSL and discontinue it as soon as feasible, starting antiviral treatment while carefully examining the patient's clinical symptoms. The PSL dose was reduced to 20 mg/day. On day 81, treatment with molnupiravir 800 mg twice daily orally for 5 days was initiated because the patient complained of dyspnea on exertion. However, on day 88, low-grade fever was observed, and chest CT revealed consolidation in both lower lobes accompanied by significant lung shrinkage on day 96. Therefore, remdesivir (200 mg on day 1, followed by 100 mg daily on days 2–10) was administered. The fever rapidly decreased, and chest CT showed regression of the bilateral consolidations in the lower lobes on day 118. However, 7 days after the administration of remdesivir, high fever recurred during PSL treatment at 10 mg/day. Additionally, molecular antigen tests were still positive with an RT-PCR Ct value of 24.09; therefore, ensitrelvir (375 mg on day 1, followed by 250 mg/day for 4 days) was administered on day 133. The CRP level remained elevated (10.35 mg/dL). However, antigenic NPS testing yielded negative results with an RT-PCR Ct value of 38.79 on day 141. The following day, the PSL dose was reduced to 5 mg/day. Notably, 1 week after completing ensitrelvir treatment, the patient developed a high fever and respiratory symptoms.
On day 153, chest CT revealed worsening of bilateral consolidation in the lower lobes (Fig. 1B). Therefore, a 10-day course of remdesivir was initiated. All symptoms subsided rapidly, and pneumonia improved. On day 173, chest radiography showed worsening of the left lung field, but the RT-PCR antigen NPS test was negative. PSL was terminated, and ensitrelvir was administered for 5 days. On day 219, the cough and high fever persisted for a few days, and chest CT revealed a marked improvement in bilateral consolidation but a new appearance of ground-glass opacities in the left upper lobe (Fig. 1C) that was negative for antigenic NPS. Therefore, ensitrelvir treatment was resumed for 5 days, and her symptoms improved quickly.
On day 238, bronchoscopy was performed, and the bronchoalveolar lavage fluid tested positive for SARS-CoV-2 using RT-PCR with a Ct value of 37.49. Blood samples showed a CRP of 3.16, and the interleukin 6 (IL-6) level was 18.0 (normal range: 0–7 pg/mL). On day 282, the patient was asymptomatic, but a chest CT showed ground-glass opacities in different areas despite improvement in the shadows observed on the previous CT scan (Fig. 1D). Blood samples had a CRP level of 2.15, an IL-6 level of 9.7, and a peripheral blood B-cell frequency of 0 %. On day 395, blood test values, including CRP and IL-6 levels, returned to normal, and a chest CT scan showed that the lung infiltrates had almost disappeared (Fig. 1E). The peripheral blood B-cell frequency was 3.3 %, indicating B-cell recovery. Approximately 1 year and 3 months had passed since the last obinutuzumab injection.
3. Discussion
FL is a mature B cell neoplasm with an indolent course. The current standard treatment for FL with a high tumor burden is a combination of obinutuzumab, an anti-CD20 monoclonal antibody, and chemotherapies, such as bendamustine or cyclophosphamide, vincristine, and prednisone, followed by obinutuzumab maintenance therapy [4]. Bendamustine, an alkylating agent, causes severe and prolonged lymphopenia and significantly decreases the number of CD4+ T lymphocytes. In this case, the CD4+ T cell count remained low (CD4+ T cell <200/μL) on day 339 amid a decrease in the lymphocyte count. However, as the lymphocyte count increased, the CD4+ T cell count also tended to increase, although it remained below 300/μL (Fig. 2). Recent studies have reported that prior exposure to bendamustine in patients with lymphoma is an independent risk factor for death and prolonged viral shedding time [5,6]. This case also suggests that bendamustine may reduce CD4+ T cell count over the long term, contributing to persistent SARS-CoV-2 infection. Moreover, obinutuzumab has a half-life of only 28 days and enhances antibody-dependent cell-mediated cytotoxicity, inducing higher levels of direct cell death than conventional rituximab [2,3], followed by humoral immunodeficiency by depleting B-lymphocytes. However, the duration of humoral immunity recovery after the completion of obinutuzumab is unclear.
The duration of viral shedding after the onset of COVID-19 is typically 20 days [7]. However, immunocompromised patients with hematologic malignancies experience prolonged viral shedding and persistent infection [8,9], especially those receiving antineoplastic chemotherapy during the previous 30 days and treatment with anti-CD20 antibodies or cellular therapy during the previous year [9]. In our patient, specific anti-SARS-CoV-2 antibodies were not detected despite a triple-dose vaccination. Onishi et al. [10] demonstrated that antibody production following vaccination requires a period exceeding 12 months from the last anti-CD20 treatment dose to vaccination. Moreover, after SARS-CoV-2 infection, the seroconversion rate is only 59 % in patients previously treated with anti-CD20 antibodies [11]. Therefore, our patient was at high risk of long-term SARS-CoV-2 infection.
Corticosteroids are immunomodulators that cause lymphopenia, especially the reduction of CD4+ T cells, and affect B cells by suppressing immunoglobulin production, activation, and proliferation. After SARS-CoV-2 infection, interstitial lung disease is often observed in survivors and may present as pulmonary post-acute sequelae of COVID-19 [12]. In these cases, a longer course of corticosteroids may be administered. In the present case, corticosteroid administration caused lymphopenia, which may have induced a disorder in cell-mediated immunity and further contributed to delayed B-cell recovery.
Whether COVID-19 can be cured without humoral immunity remains unknown. Soresina et al. [13] concluded that B cell function might be dispensable in the immune response against SARS-CoV-2 and that the remaining T-cell function might be sufficient to overcome infection. However, the complete elimination of SARS-CoV-2 from the lungs was not confirmed. The resolution of SARS-CoV-2 infection has been reported in patients with FL receiving B-cell depletion therapy. A decrease in the CD4+ T-cell count, compensatory innate immunity, and CD8+ T-cell activation combined with the loss of B-cell function can enable the control of acute SARS-CoV-2 infection and/or viremia but may be insufficient to achieve adequate viral clearance without the requisite level of neutralizing antibodies [8]. In this case, diffuse pulmonary ground-glass opacification persisted despite the patient being asymptomatic, with repeated viral negativity in the NPS while bronchoalveolar lavage fluid was PCR-positive for SARS-CoV-2. Because the B-cell frequency increased and clinical blood values and chest CT images improved, the infection was considered resolved.
Here, we report a case of SARS-CoV-2 infection in a patient with impaired humoral immunity. In such cases, long-term administration of corticosteroids may induce cellular immunodeficiency, resulting in prolonged SARS-CoV-2 infection. One year and three months after the administration of obinutuzumab, B-cell recovery was observed, and the infection had resolved. Without the recovery of humoral immunity, SARS-CoV-2 infection may be difficult to overcome.
CRediT authorship contribution statement
Yuriko Sueda: Writing – original draft. Hirokazu Tokuyasu: Writing – review & editing, Conceptualization. Momoka Atsuta: Investigation. Hiromitsu Sakai: Investigation. Katsunori Arai: Investigation. Chika Esumi: Investigation, Validation. Misato Mochizuki: Resources. Tomoki Itohara: Investigation, Validation. Naoki Fujisawa: Investigation, Validation. Akira Yamasaki: Writing – review & editing.
4. Funding
No funding was received for this work.
Declaration of competing interest
The authors declare that they have no conflicts of interest.
Acknowledgments
The authors thank Editage for their assistance with English language editing.
References
- 1.Yigenoglu T.N., Ata N., Altuntas F., Bascı S., Dal M.S., Korkmaz S., et al. The outcome of COVID-19 in patients with hematological malignancy. J. Med. Virol. 2021;93:1099–1104. doi: 10.1002/jmv.26404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies A., Kater A.P., Sharman J.P., Stilgenbauer S., Vitolo U., Klein C., et al. Obinutuzumab in the treatment of B-cell malignancies: a comprehensive review. Future Oncol. 2022;18:2943–2966. doi: 10.2217/fon-2022-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salles G., Barrett M., Foà R., Maurer J., O'Brien S., Valente N., et al. Rituximab in B-cell hematologic malignancies: a review of 20 years of clinical experience. Adv. Ther. 2017;34:2232–2273. doi: 10.1007/s12325-017-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcus R., Davies A., Ando K., Klapper W., Opat S., Owen C., et al. Obinutuzumab for the first-line treatment of follicular lymphoma. N. Engl. J. Med. 2017;377:1331–1344. doi: 10.1056/NEJMoa1614598. [DOI] [PubMed] [Google Scholar]
- 5.Assanto G.M., Di Rocco A., Malfona F., Capriata M., Del Giudice I., Petrucci L., et al. Impact of anti-SARS-CoV-2 monoclonal antibodies in the management of patients with lymphoma and COVID19: a retrospective study. Hematol. Oncol. 2023;41:343–353. doi: 10.1002/hon.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maruyama S., Wada D., Kanayama S., Shimazu H., Miyano Y., Inoue A., et al. The evaluation of risk factors for prolonged viral shedding during anti-SARS-CoV-2 monoclonal antibodies and long-term administration of antivirals in COVID-19 patients with B-cell lymphoma treated by anti-CD20 antibody. BMC Infect. Dis. 2024;24:715. doi: 10.1186/s12879-024-09631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young B.E., Ong S.W.X., Kalimuddin S., Low J.G., Tan S.Y., Loh J., et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323:1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deveci B., Saba R. Prolonged viral positivity induced recurrent coronavirus disease 2019 (COVID-19) pneumonia in patients receiving anti-CD20 monoclonal antibody treatment: case reports. Medicine (Baltim.) 2021;100 doi: 10.1097/MD.0000000000028470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee C.Y., Shah M.K., Hoyos D., Solovyov A., Douglas M., Taur Y., et al. Prolonged SARS-CoV-2 infection in patients with lymphoid malignancies. Cancer Discov. 2022;12:62–73. doi: 10.1158/2159-8290.CD-21-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onishi A., Matsumura-Kimoto Y., Mizutani S., Tsukamoto T., Fujino T., Miyashita A., et al. Impact of treatment with anti-CD20 monoclonal antibody on the production of neutralizing antibody against anti-SARS-CoV-2 vaccination in mature B-cell neoplasms. Infect. Drug Resist. 2023;16:509–519. doi: 10.2147/IDR.S396271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thakkar A., Pradhan K., Jindal S., Cui Z., Rockwell B., Shah A.P., et al. Patterns of seroconversion for SARS-CoV-2- IgG in patients with malignant disease and association with anticancer therapy. Nat. Can. 2021;2:392–399. doi: 10.1038/s43018-021-00191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stewart I., Jacob J., George P.M., Molyneaux P.L., Porter J.C., Allen R.J., et al. Residual lung abnormalities after COVID-19 hospitalization: interim analysis of the UKILD post-COVID-19 study. Am. J. Respir. Crit. Care Med. 2023;207:693–703. doi: 10.1164/rccm.202203-0564OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soresina A., Moratto D., Chiarini M., Paolillo C., Baresi G., Focà E., et al. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover, Pediatr. Allerg. Immunol. 2020;31:565–569. doi: 10.1111/pai.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]