ABSTRACT
Enterococcus faecalis and Enterococcus faecium are frequent causes of healthcare-associated infections. Antimicrobial-resistant enterococci pose a serious public health threat, particularly vancomycin-resistant enterococci (VRE), for which treatment options are limited. The Centers for Disease Control and Prevention’s Division of Healthcare Quality Promotion Sentinel Surveillance system conducted surveillance from 2018 to 2019 to evaluate antimicrobial susceptibility profiles and molecular epidemiology of 205 E. faecalis and 180 E. faecium clinical isolates collected from nine geographically diverse sites in the United States. Whole genome sequencing revealed diverse genetic lineages, with no single sequence type accounting for more than 15% of E. faecalis or E. faecium. Phylogenetic analysis distinguished E. faecium from 19 E. lactis (previously known as E. faecium clade B). Resistance to vancomycin was 78.3% among E. faecium, 7.8% among E. faecalis, and did not occur among E. lactis isolates. Resistance to daptomycin and linezolid was rare: E. faecium (5.6%, 0.6%, respectively), E. faecalis (2%, 2%), and E. lactis (5.3%, 0%). All VRE harbored the vanA gene. Three of the seven isolates that were not susceptible to linezolid harbored optrA, one chromosomally located and two on linear plasmids that shared a conserved backbone with other multidrug-resistant conjugative linear plasmids. One of these isolates contained optrA and vanA co-localized on the linear plasmid. By screening all enterococci, 20% of E. faecium were predicted to harbor linear plasmids, whereas none were predicted among E. faecalis or E. lactis. Continued surveillance is needed to assess the future emergence and spread of antimicrobial resistance by linear plasmids and other mechanisms.
IMPORTANCE
This work confirms prior reports of E. faecium showing higher levels of resistance to more antibiotics than E. faecalis and identifies that diverse sequence types are contributing to enterococcal infections in the United States. All VRE harbored the vanA gene. We present the first report of the linezolid resistance gene optrA on linear plasmids in the United States, one of which co-carried a vanA cassette. Additional studies integrating epidemiological, antimicrobial susceptibility, and genomic methods to characterize mechanisms of resistance, including the role of linear plasmids, will be critical to understanding the changing landscape of enterococci in the United States.
KEYWORDS: Enterococcus faecalis, Enterococcus faecium, daptomycin, linezolid, optrA, linear plasmid
INTRODUCTION
Enterococci, including Enterococcus faecalis and Enterococcus faecium, are frequently reported pathogens responsible for healthcare-associated infections (HAIs). The Centers for Disease Control and Prevention (CDC) deemed vancomycin-resistant enterococci (VRE), which account for ~30% of healthcare-associated enterococcal infections, a serious public health threat, particularly because treatment options are limited, especially for vancomycin-resistant E. faecium (1, 2). There were an estimated 54,457 VRE infections in hospitalized patients in 2017 (1, 3). Although VRE infections decreased from 84,800 to 54,500 between 2012 and 2017, the rate of VRE infections increased 16% from 2019 to 2020 during the COVID-19 pandemic (1, 3, 4). Increased vigilance and further action are needed to reverse this increase.
Linezolid and daptomycin are antibiotics used for the treatment of infections caused by VRE. Although rates of resistance to linezolid and daptomycin among US enterococci are low (5), resistance to these antibiotics has emerged (5, 6). Resistance to linezolid is associated with the presence of mutations in the V domain of the 23S rRNA gene or the acquisition of genes encoding resistance to oxazolidinones, including cfr, cfr(B), cfr(D), optrA, and poxtA (7–9). Mutations in the liaFSR operon that encode a 3-component system that regulates cell envelope integrity, and mutations in cls that encodes cardiolipin synthase, are associated with daptomycin resistance (10). However, resistance to daptomycin is multifactorial, and the presence of mutations in liaFSR and cls alone may not always result in elevated MICs and additional mechanisms exist (11–15).
Surveillance data are crucial in our efforts to combat resistance; strengthening surveillance is one of the US Government’s goals in the National Strategy for Combating Antibiotic-Resistant Bacteria (Federal Task Force on Combating Antibiotic-Resistant Bacteria) (16). Antimicrobial susceptibility testing (AST) using reference methods generates helpful and necessary phenotypic surveillance data (17–19); however, molecular surveillance is also needed to detect and characterize emerging resistance mechanisms and identify dominant strains in the healthcare setting. For example, the spread of vancomycin-resistant E. faecium isolates with pstS-null sequence types has been reported internationally, and the ST1478 pstS-null sequence type associated with increased daptomycin nonsusceptibility was reported to have rapidly disseminated across acute care hospitals in Canada between 2013 and 2018 (20–23). However, there is a lack of data available to determine if pstS-null sequence types are circulating in the United States.
The U.S. CDC Division of Healthcare Quality Promotion (DHQP) Sentinel Surveillance system studies the antimicrobial resistance profiles and resistance mechanisms of a convenience sample of bacteria collected annually from hospitalized patients from geographically diverse facilities in the United States. Sentinel Surveillance isolates are selected without regard to antimicrobial susceptibility profile (i.e., both resistant and susceptible isolates). The CDC performed surveillance for E. faecalis and E. faecium using the DHQP Sentinel Surveillance system between 2018 and 2019. Isolates were characterized using both reference AST and whole genome sequencing (WGS) to provide insights into the in vitro activity of various antibiotics used to treat enterococci, describe the molecular epidemiology of both vancomycin-susceptible and -resistant isolates at nine sites in the United States, and describe the molecular mechanisms of resistance observed.
RESULTS
Bacterial isolate collection and identification
During the 2018–2019 collection period, 404 enterococci were included in this study from nine different facilities across the United States (Table 1). Of the 205 isolates submitted as E. faecalis, all were identified as E. faecalis by matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS) (Table S1). Of the 199 isolates submitted as E. faecium, 190/199 isolates were identified as E. faecium by MALDI-TOF MS. There were nine of 199 isolates that were submitted as E. faecium that were identified as E. lactis by MALDI-TOF MS. Whole genome sequencing was performed on all isolates. Average nucleotide identity (ANI) was performed, and four of 199 isolates submitted as E. faecium were identified as E. lactis. Ultimately, phylogenetics was used to distinguish between closely related E. faecium and E. lactis, discussed in greater detail below. A total of 180 isolates were identified as E. faecium clade A, and 19 isolates were identified as E. lactis (previously known as E. faecium clade B) by phylogenetic separation (Fig. S1). Enterococci were isolated from urine (n = 168, 41.6%), blood (n = 130, 32.2%), and other sources (n = 106, 26.2%) including wounds, tissues, and body fluids (Table S1).
TABLE 1.
Number of isolates and demographicsa
| E. faecalis (n = 205) | E. faecium (n = 180) | E. lactis (n = 19) | |
|---|---|---|---|
| Source | |||
| Blood | 63 (30.7%) | 60 (33.3%) | 7 (36.8%) |
| Urine | 91 (44.4%) | 71 (39.4%) | 6 (31.6%) |
| Other | 51 (24.9%) | 49 (27.2%) | 6 (31.6%) |
| Site | |||
| CA | 21 (10.2%) | 17 (9.4%) | 2 (10.5%) |
| IA | 26 (12.7%) | 26 (14.4%) | - |
| MD | 21 (10.2%) | 16 (8.9%) | 2 (10.5%) |
| MN | 14 (6.8%) | 12 (6.7%) | 2 (10.5%) |
| NM | 20 (9.8%) | 18 (10.0%) | 1 (5.3%) |
| NY | 24 (11.7%) | 24 (13.3%) | 4 (21.1%) |
| NC | 27 (13.2%) | 16 (8.9%) | 2 (10.5%) |
| PA | 27 (13.2%) | 18 (10.0%) | 2 (10.5%) |
| WA | 25 (12.2%) | 33 (18.3%) | 4 (21.1%) |
| Multi-locus sequence types (STs) | ST179 (n = 26); ST16 (n = 20); ST40 (n = 20); ST6 (n = 19); ST21 (n = 7); ST64 (n = 7); ST109 (n = 6); ST103 (n = 5); ST778 (n = 5); others (n = 90) | ST80 (n = 26); ST17 (n = 23); ST18 (n = 22); ST412 (n = 21); ST117 (n = 18); ST736 (n = 9); ST78 (n = 6); ST1478 (n = 5); others (n = 50) | ST240 (n = 4); ST94 (n = 3); ST800 (n = 2); others (n = 10) |
| Patient age (median) | 64 | 61 | 57 |
CA, California; IA, Iowa; MD, Maryland; MN, Minnesota; NM, New Mexico; NY, New York; NC, North Carolina; PA, Pennsylvania; WA, Washington. - indicates no E. lactis isolates were received from that site.
Antimicrobial susceptibility testing
A summary of the susceptibility profiles of E. faecalis, E. faecium, and E. lactis isolates is presented (Table 2). E. faecium MIC susceptibility test interpretive criteria (breakpoints) were used for E. lactis isolates for the purposes of categorical interpretation due to the genomic similarity between E. faecium and E. lactis (24). All E. faecalis and E. lactis were susceptible to ampicillin and penicillin, but most E. faecium isolates were resistant to both (n = 167, 92.8%). Resistance to vancomycin was uncommon among E. faecalis isolates (n = 16, 7.8%) but common among E. faecium (n = 141, 78.3%). No E. lactis isolates displayed vancomycin resistance. Resistance to daptomycin was rare, with 2.0% (4/205) of E. faecalis, 5.6% (10/180) of E. faecium, and 5.3% (1/19) of E. lactis isolates displaying resistance. Resistance to linezolid was also rare with 2.0% (4/205) of E. faecalis, 0.6% (1/180) of E. faecium, and no E. lactis isolates displaying resistance. Three vancomycin-resistant E. faecalis displayed resistance to linezolid and intermediate susceptibility to daptomycin. Two vancomycin-resistant E. faecium displayed resistance or intermediate susceptibility to linezolid; both were categorized as susceptible-dose dependent (SDD) to daptomycin. It should be noted that the Clinical and Laboratory Standards Institute (CLSI)-adopted SDD breakpoint for E. faecium is based on a dosage of 8–12 mg/kg, which is higher than the Food and Drug Administration (FDA)-approved dose (25).
TABLE 2.
Antimicrobial susceptibility testing results for E. faecalis, E. faecium, and E. lactis, including vancomycin-resistant E. faecalis and E. faecium, 2018–2019 DHQP Sentinel Surveillance system isolatesa
| % Susceptible | % Intermediate | % Resistant | MIC range (µg/mL) | MIC50 (µg/mL) | MIC90 (µg/mL) | |
|---|---|---|---|---|---|---|
| E. faecalis (n = 205) | ||||||
| Ampicillin | 100.0 | N/A | 0.0 | ≤0.25 to 2 | 1 | 1 |
| Penicillin | 100.0 | N/A | 0.0 | ≤0.25 to 8 | 2 | 4 |
| Vancomycin | 92.2 | 0.0 | 7.8 | 0.5 to > 64 | 1 | 2 |
| Teicoplanin | 92.2 | 0.0 | 7.8 | ≤0.12 to > 64 | 0.5 | 1 |
| Daptomycin | 80.0 | 18.0 | 2.0 | ≤0.25 to 16 | 2 | 4 |
| Linezolid | 98.0 | 0.0 | 2.0 | ≤0.5 to 16 | 2 | 2 |
| Tedizolidb | 98.0 | N/A | 2.0 | ≤0.12 to 2 | 0.5 | 0.5 |
| Quinupristin/dalfopristinc | N/A | N/A | N/A | 0.5 to > 8 | 8 | 8 |
| Levofloxacin | 77.1 | 0.0 | 22.9 | 0.5 to > 8 | 1 | >8 |
| Doxycycline | 45.9 | 47.3 | 6.8 | ≤2 to 32 | 8 | 8 |
| Tigecyclined | 100.0 | N/A | N/A | 0.06 to 0.25 | 0.12 | 0.12 |
| Eravacyclineb,e | 95.1 | N/A | 4.9 | ≤0.016 to 0.12 | 0.06 | 0.06 |
| Chloramphenicol | 89.3 | 1.5 | 9.3 | ≤2 to > 32 | 4 | 16 |
| Gentamicinf | 77.6 | N/A | 22.4 | - | - | - |
| Streptomycinf | 83.4 | N/A | 16.6 | - | - | - |
| VR E. faecalis (n = 16) | ||||||
| Ampicillin | 100.0 | N/A | 0.0 | 0.5 to 2 | 1 | 1 |
| Penicillin | 100.0 | N/A | 0.0 | 2 to 8 | 4 | 4 |
| Vancomycin | 0.0 | 0.0 | 100.0 | >64 | >64 | >64 |
| Teicoplanin | 0.0 | 0.0 | 100.0 | 32 to > 64 | >64 | >64 |
| Daptomycin | 75.0 | 18.8 | 6.3 | 0.5 to 8 | 1 | 4 |
| Linezolid | 81.3 | 0.0 | 18.8 | ≤0.5 to 16 | 2 | 16 |
| Tedizolidb | 81.3 | N/A | 18.8 | ≤0.12 to 2 | 0.25 | 2 |
| Quinupristin/dalfopristinc | N/A | N/A | N/A | 0.5 to > 8 | 8 | 8 |
| Levofloxacin | 0.0 | 0.0 | 100.0 | 8 to > 8 | >8 | >8 |
| Doxycycline | 75.0 | 25.0 | 0.0 | ≤2 to 8 | 4 | 8 |
| Tigecyclined | N/A | N/A | N/A | 0.06 to 0.25 | 0.12 | 0.12 |
| Eravacyclineb,e | 93.8 | N/A | 6.3 | ≤0.016 to 0.12 | 0.06 | 0.06 |
| Chloramphenicol | 62.5 | 18.8 | 18.8 | 8 to > 32 | 8 | >32 |
| Gentamicinf | 18.8 | N/A | 81.3 | - | - | - |
| Streptomycinf | 68.8 | N/A | 31.3 | - | - | - |
| E. faecium (n = 180) | ||||||
| Ampicillin | 7.2 | N/A | 92.8 | ≤0.25 to > 32 | >32 | >32 |
| Penicillin | 6.7 | N/A | 93.3 | ≤0.25 to > 32 | >32 | >32 |
| Vancomycin | 21.1 | 0.6 | 78.3 | 0.5 to > 64 | >64 | >64 |
| Teicoplanin | 21.1 | 0.6 | 78.3 | 0.5 to > 64 | >64 | >64 |
| Daptomycing | 94.4 | N/A | 5.6 | ≤0.25 to 16 | 4 | 4 |
| Linezolid | 98.3 | 1.1 | 0.6 | ≤0.5 to 8 | 2 | 2 |
| Tedizolid | N/A | N/A | N/A | ≤0.12 to 1 | 0.5 | 0.5 |
| Quinupristin/dalfopristinh | N/A | N/A | N/A | 0.25 to 8 | 1 | 2 |
| Levofloxacin | 5.0 | 2.8 | 92.2 | 1 to > 8 | >8 | >8 |
| Doxycycline | 50.6 | 13.9 | 35.6 | ≤2 to 32 | 4 | 16 |
| Tigecyclined | N/A | N/A | N/A | ≤0.03 to 0.5 | 0.06 | 0.12 |
| Eravacyclineb,e | 88.3 | N/A | 11.7 | ≤0.016 to > 0.25 | 0.06 | 0.12 |
| Chloramphenicol | 87.2 | 11.1 | 1.7 | 4 to > 32 | 8 | 16 |
| Gentamicinf | 88.9 | N/A | 11.1 | - | - | - |
| Streptomycinf | 60.6 | N/A | 39.4 | - | - | - |
| VR E. faecium (n = 141) | ||||||
| Ampicillin | 0.0 | N/A | 100.0 | 32 to > 32 | >32 | >32 |
| Penicillin | 0.0 | N/A | 100.0 | >32 | >32 | >32 |
| Vancomycin | 0.0 | 0.0 | 100.0 | >64 | >64 | >64 |
| Teicoplanin | 0.0 | 0.7 | 99.3 | 16 to > 64 | >64 | >64 |
| Daptomycing | 95.7 | N/A | 4.3 | ≤0.25 to 16 | 4 | 4 |
| Linezolid | 98.6 | 0.7 | 0.7 | ≤0.5 to 8 | 2 | 2 |
| Tedizolid | N/A | N/A | N/A | ≤0.12 to 1 | 0.5 | 0.5 |
| Quinupristin/dalfopristinh | 89.4 | 3.5 | 7.1 | 0.25 to 8 | 1 | 2 |
| Levofloxacin | 0.0 | 0.0 | 100.0 | >8 | >8 | >8 |
| Doxycycline | 52.5 | 13.5 | 34.0 | ≤2 to 32 | 4 | 16 |
| Tigecyclined | N/A | N/A | N/A | ≤0.03 to 0.5 | 0.06 | 0.12 |
| Eravacyclineb, e | 88.7 | N/A | 11.3 | ≤0.016 to > 0.25 | 0.06 | 0.12 |
| Chloramphenicol | 87.2 | 11.3 | 1.4 | 4 to > 32 | 8 | 16 |
| Gentamicinf | 90.8 | N/A | 9.2 | - | - | - |
| Streptomycinf | 58.9 | N/A | 41.1 | - | - | - |
| E. lactis (n = 19) | ||||||
| Ampicillin | 100.0 | N/A | 0.0 | ≤0.25 to 2 | 1 | 2 |
| Penicillin | 100.0 | N/A | 0.0 | ≤0.25 to 8 | 4 | 8 |
| Vancomycin | 100.0 | 0.0 | 0.0 | 0.5 to 1 | 0.5 | 1 |
| Teicoplanin | 100.0 | 0.0 | 0.0 | 0.5 to 2 | 1 | 1 |
| Daptomycing | 94.7 | N/A | 5.3 | 0.5 to 8 | 4 | 4 |
| Linezolid | 100.0 | 0.0 | 0.0 | 1 to 2 | 2 | 2 |
| Tedizolid | N/A | N/A | N/A | 0.25 to 0.5 | 0.5 | 0.5 |
| Quinupristin/dalfopristinh | N/A | N/A | N/A | 1 to 4 | 2 | 2 |
| Levofloxacin | 89.5 | 5.3 | 5.3 | 0.5 to > 8 | 1 | 4 |
| Doxycycline | 94.7 | 0.0 | 5.3 | ≤2 to 16 | ≤2 | ≤2 |
| Tigecyclined | N/A | N/A | N/A | 0.06 | 0.06 | 0.06 |
| Eravacyclineb, e | 100.0 | N/A | 0.0 | 0.03 to 0.06 | 0.06 | 0.06 |
| Chloramphenicol | 94.7 | 5.3 | 0.0 | 4 to 16 | 4 | 8 |
| Gentamicinf | 100.0 | N/A | 0.0 | - | - | - |
| Streptomycinf | 94.7 | N/A | 5.3 | - | - | - |
N/A, isolates were tested, but no breakpoints were available; VR, vancomycin resistant.
For tedizolid and eravacycline, % nonsusceptible is reported as there is no resistant category.
E. faecalis is intrinsically resistant to quinupristin/dalfopristin.
U.S. FDA MIC susceptibility test interpretive criteria (breakpoint) for tigecycline (susceptible ≤0.25 μg/mL) is only available for vancomycin-susceptible E. faecalis. The FDA breakpoint for tigecycline was only applied to vancomycin-susceptible E. faecalis isolates (n=189). CLSI breakpoints are not established.
U.S. FDA MIC susceptibility test interpretive criteria (breakpoint) for eravacycline (susceptible ≤0.06 μg/mL) was used as CLSI breakpoints are not established.
E. faecalis and E. faecium isolates are intrinsically resistant to aminoglycosides. High-level gentamicin resistance and high-level streptomycin resistance were tested using 500 µg/mL and 1000 µg/mL of the antibiotic, respectively. Susceptibility implies synergy between the aminoglycoside and the cell wall active agent and resistance implies no synergy between the aminoglycoside and the cell wall active agent; therefore, MIC range, MIC50, and MIC90 are indicated as (-).
For daptomycin and E. faecium/E.lactis, there is no susceptible breakpoint so susceptible-dose dependent (SDD) is reported. Given the close genetic relationship between E. faecium and E. lactis, E. faecium daptomycin breakpoints were applied to the E. lactis isolates.
For quinupristin/dalfopristin, CLSI guidance is to report only on vancomycin-resistant E. faecium.
Whole genome sequencing, phylogenetics and MLST distribution
All isolates underwent WGS, and to better understand the diversity within each species and the relationship between genetic relatedness, resistance, and demographics, we performed multi-locus sequence typing (MLST) and core genome phylogenetic reconstruction and compared with AST. A summary of sequence types (STs) is provided in Table 1. Overall, STs for E. faecalis, E. faecium, and E. lactis were highly diverse: for E. faecalis, the Simpson’s Diversity Index was 0.95; for E. faecium, it was 0.92; and for E. lactis, it was 0.89.
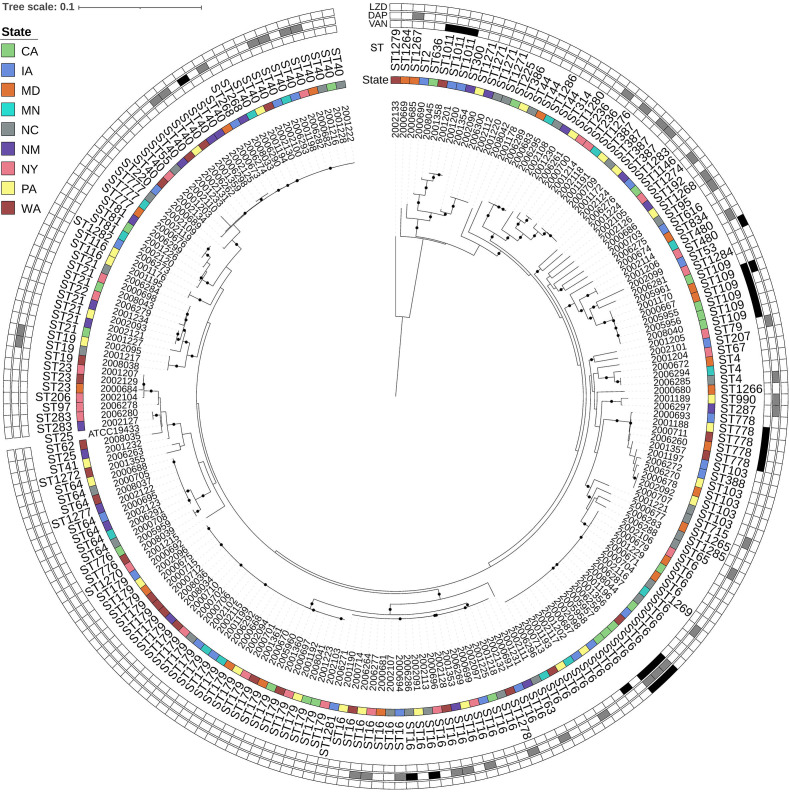
E. faecalis did not cluster phylogenetically within collection sites, and 73 STs were represented (Fig. 1). Vancomycin resistance was identified in multiple sites within a few E. faecalis STs: ST109 (n = 5), ST778 (n = 4), ST6 (n = 4), and ST1011 (n = 3). Daptomycin resistance was identified infrequently among ST16 (n = 2), ST109 (n = 1), and ST40 (n = 1), whereas daptomycin-intermediate E. faecalis were spread across many different STs. Linezolid resistance was identified in ST6 (n = 3) and ST480 (n = 1) isolates (Table S1).
Fig 1.
E. faecalis maximum likelihood phylogeny based on core gene alignments generated by Roary and RaxML. Annotation rings (from inner to outer) show isolate ID, state of origin, multi-locus sequence type (ST), minimum inhibitory concentration (MIC) interpretations for vancomycin, daptomycin, and linezolid. ST obtained using E. faecalis scheme from PubMLST, MIC interpretations represent susceptible in white; intermediate in gray; and resistant in black. Black circles on branches represent 100% support for the branch out of 100 bootstraps, and the tree is rooted at the midpoint. Abbreviations: LZD, linezolid; DAP, daptomycin; VAN, vancomycin; ST; multi-locus sequence type, CA, California; IA, Iowa; MD, Maryland; MN, Minnesota; NM, New Mexico; NY, New York; NC, North Carolina; PA, Pennsylvania; and WA, Washington.
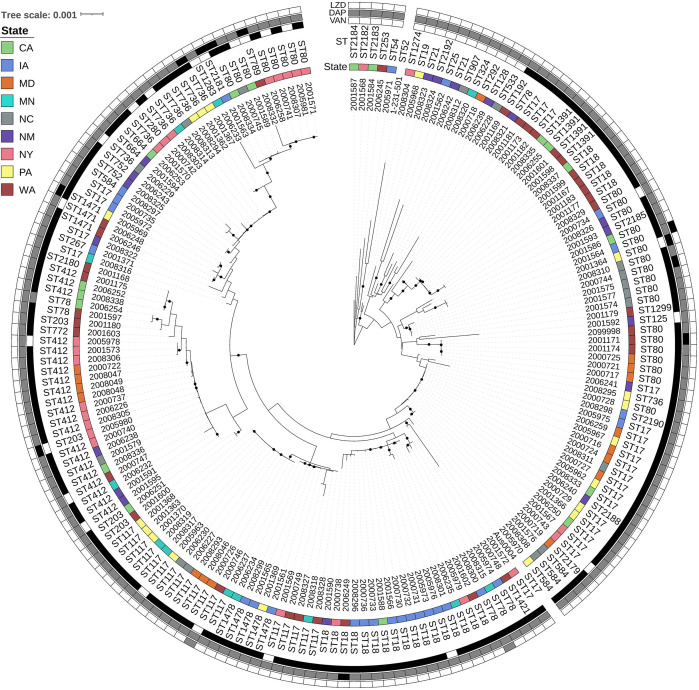
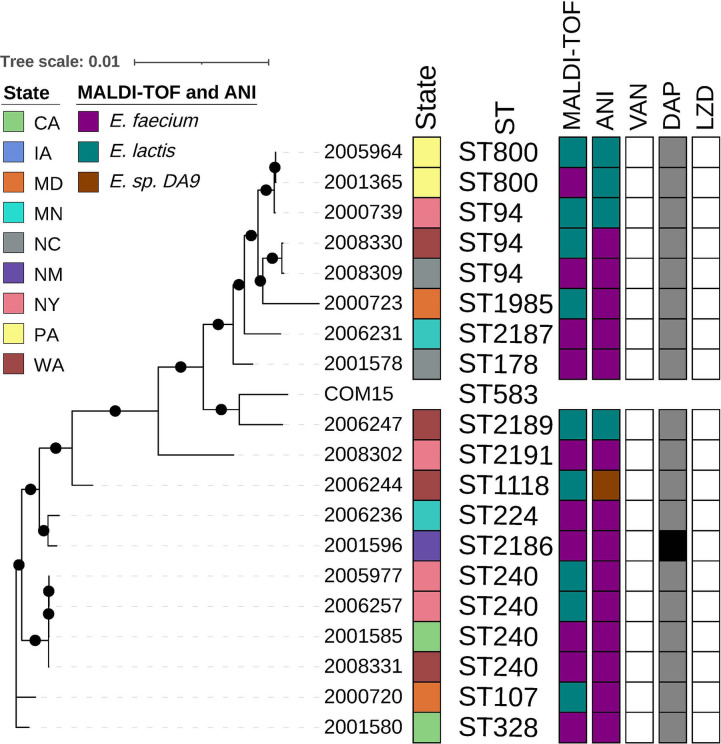
Performing a phylogenetic analysis of isolates submitted as E. faecium, we observed two clades, A (Fig. 2) with n = 180 isolates with 44 STs and B (Fig. 3) with n = 19 isolates with 13 STs, identified by previously established reference isolates Aus0004 (26) and COM15 (27), respectively (28). Vancomycin resistance was identified throughout the E. faecium tree across 24 STs with the most in the most prevalent STs, for example, ST17 (n = 22), ST18 (n = 21), and ST80 (n = 21) (Fig. 2). Vancomycin resistance was not present in any E. lactis isolate (Fig. 3). Daptomycin resistance was also distributed across several STs throughout E. faecium (Fig. 2), with the following STs represented ST80 (n = 4), ST18 (n = 1), ST203 (n = 1), ST736 (n = 1), ST789 (n = 1), ST1471 (n = 1), and ST2181 (n = 1). A single E. lactis isolate, ST2186, displayed daptomycin resistance. Linezolid resistance in E. faecium was found only in ST80 (n = 1), and linezolid-intermediate isolates belonged to ST78 (n = 1) and ST117 (n = 1). One linezolid-resistant (ST80) isolate and one linezolid-intermediate (ST78) isolate were also resistant to vancomycin; both were categorized as SDD to daptomycin.
Fig 2.
E. faecium maximum likelihood phylogeny based on core gene alignments generated by Roary and RaxML. Annotation rings (from inner to outer) show isolate ID, state of origin, multi-locus sequence type (ST), minimum inhibitory concentration (MIC) interpretations for vancomycin, daptomycin, and linezolid. ST obtained using E. faecium scheme from PubMLST, MIC interpretations following E. faecium guidance represents susceptible – white; susceptible dose-dependent – gray; and resistant – black. Black circles on branches represent 100% support for the branch out of 100 bootstraps. Abbreviations: LZD, linezolid; DAP, daptomycin; VAN, vancomycin; ST, multi-locus sequence type; CA, California; IA, Iowa; MD, Maryland; MN, Minnesota; NM, New Mexico; NY, New York; NC, North Carolina; PA, Pennsylvania; and WA, Washington.
Fig 3.
E. lactis maximum likelihood phylogeny based on core gene alignments generated by Roary and RaxML. Annotation (from left to right) shows isolate ID, state of origin, multi-locus sequence type (ST), MALDI-TOF MS identified species, ANI identified species, minimum inhibitory concentration (MIC) interpretations for vancomycin, daptomycin, and linezolid. ST obtained using E. faecium scheme from PubMLST, MIC interpretations following E. faecium guidance represents susceptible in white; susceptible dose-dependent in gray; and resistant in black. Black circles on branches represent 100% support for the branch out of 100 bootstraps. Abbreviations: LZD, linezolid; DAP, daptomycin; VAN, vancomycin; ST, multi-locus sequence type; CA, California; IA, Iowa; MD, Maryland; MN, Minnesota; NM, New Mexico; NY, New York; NC, North Carolina; PA, Pennsylvania; and WA, Washington.
We assessed the distribution of the rapidly spreading and global pstS-null STs (20) (strains that are nontypable due to the absence of the pstS locus) and found that 6/141 (4.3%) vancomycin-resistant E. faecium contained the pstS-null ST, including one ST1421 (WA; SDD to daptomycin MIC 2 µg/mL) and 5 ST1478 (PA, IA, and MN; all daptomycin SDD with MICs of 2 or 4 µg/mL). When compared phylogenetically to global isolates using a core gene analysis, the ST1421 isolates clustered closely with isolates from Australia (29). However, the 5 US ST1478 were more similar in general to each other [40–115 core gene single nucleotide polymorphisms (SNPs)] than the rest of the Canadian isolates (23) (150–1897 core SNPs) except for one Canadian isolate (ID:01A18435), which ranged between 74 and 108 core SNPs to the 5 US ST1478 (Fig. S2).
Antimicrobial resistance gene and mutation analyses
Sixteen (7.8%) of the 205 E. faecalis were resistant to vancomycin; all 16 harbored the vanA resistance gene. The remaining 189 vancomycin-susceptible isolates did not contain known mechanisms associated with vancomycin resistance. Among the E. faecium, 141 (78.3%) of 180 were resistant to vancomycin and all harbored vanA. One vancomycin-intermediate (MIC 8 µg/mL) E. faecium harbored vanA. Thirty-eight (21.1%) of 180 E. faecium isolates were vancomycin susceptible and did not harbor a known vancomycin resistance gene.
Of the four E. faecalis isolates showing resistance to daptomycin (MIC ≥8 µg/mL), only one isolate (sample ID: 2006286, MIC 16 µg/mL) contained the LiaF amino acid insertion insI177 previously associated with resistance (Table S1) (30). No other genes containing mutations previously associated with daptomycin resistance (e.g., cls, gdpD, gshF, liaFSR, and yybT) (15, 30), were identified among the remaining 201 E. faecalis isolates. Of the 10 E. faecium isolates showing resistance to daptomycin (MIC ≥8 µg/mL), five contained mutations previously associated with resistance, including isolates 2006249 (Cls R218Q), 2005969 (Cls A20P, LiaR W73C, LiaS T120A), and 2001563, 2008334, 2001367 (LiaR W73C, LiaS T120A), and five did not have any genes containing mutations previously associated with daptomycin resistance [e.g., cfa, cls, divIVA, dltABCD, gdpD, gshF, liaFSR, mprF, rpoB, and yycFG (5, 10–12, 15)]. One E. lactis isolate showed resistance to daptomycin but did not harbor genes containing mutations previously associated with daptomycin resistance. Among the 170 daptomycin SDD E. faecium isolates (MIC ≤4 µg/mL), 32 (17.8%) isolates (MIC range: 2–4 µg/mL) harbored mutations previously associated with resistance, including 2005978 with Cls R218Q, LiaR W73C, LiaS T120A, 2000749 with LiaR W73C, and 30 samples with LiaR W73C, LiaS T120A (12, 15). The remaining 138 SDD isolates did not contain mutations previously associated with daptomycin resistance (5, 10–12, 15).
Of the four linezolid-resistant E. faecalis, three ST6 isolates contained the 23S rRNA G2576T mutation and one ST480 isolate contained optrA. One E. faecium isolate was linezolid-resistant and two were intermediate (MIC 4 µg/mL). One of the linezolid-intermediate isolates neither harbored a 23S rRNA mutation nor did it carry optrA, cfr, cfr(B), cfr(D), or poxtA. The remaining linezolid-resistant and linezolid-intermediate isolates contained optrA. Of the 177 linezolid-susceptible E. faecium isolates, three carried cfr(B).
optrA locations and linear plasmids
To analyze the genomic context of the three optrA-positive enterococci, the isolates were sequenced using both short- and long-read sequencing to create annotated hybrid assemblies. In E. faecalis isolate 2001206, optrA was chromosomally encoded on a Tn6674-like transposon. The 60 kb region in 2001206 surrounding optrA was similar to several publicly available genomes from the National Center for Biotechnology Information (NCBI) with high identity (>99.2%) and perfect coverage including EFS17 (Accession CP085289), JF31-223 (Accession CP102065), JARB-HU0796 (Accession AP027136), and AT40a (Accession CP097069). The Tn6674-like region in 2001206 had 99.8% identity in the overlapping regions of Tn6674 [Accession MK737778 (31)] with an ~900 bp internal deletion that did not appear to interrupt any coding genes. OptrA is located on this transposon in addition to resistance genes spc, erm(A), and fexA. In the two E. faecium isolates, 2001564 and 2008300, we identified optrA on plasmids that did not circularize with sizes of 114,744 bp and 106,518 bp, respectively.
S1 nuclease pulsed-field gel electrophoresis (PFGE) was used to assess plasmid topology. S1 nuclease, which converts supercoiled plasmid DNA into linear molecules, was used to compare digested total DNA to undigested total DNA for all three optrA-positive isolates. Linear plasmids were not identified in 2001206; however, linear fragments of sizes ~114 kb and ~106 kb were observed in S1 nuclease-digested and undigested samples for isolates 2001564 and 2008300, respectively, which is characteristic of a plasmid with linear topology (32) (Fig. 4). The linear plasmids correspond approximately in size to the assembled contigs carrying optrA as identified with WGS analysis. Following the nomenclature pELF, enterococcal linear form plasmid (33), the plasmids were designated pELF_2001564 and pELF_2008300.
Fig 4.
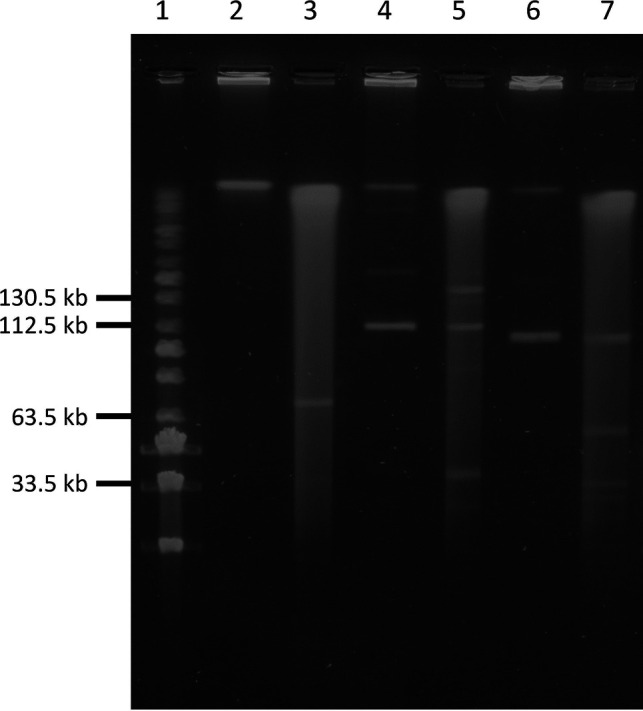
Confirmation of the presence of linear plasmids by S1 nuclease pulsed-field gel electrophoresis (PFGE) of optrA-positive enterococci. Lane 1, PFG MidRange Marker; lane 2, 2001206 (undigested); lane 3, 2001206 (digested); lane 4, 2001564 (undigested, ~114 kb linear plasmid present); lane 5, 2001564 (digested, ~114 kb linear plasmid present); lane 6, 2008300 (undigested, ~106 kb plasmid present); and lane 7, 2008300 (digested, ~106 kb plasmid present).
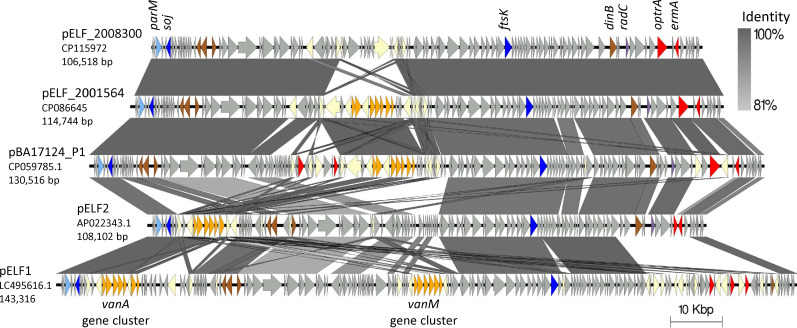
We compared the two plasmids with one another and other known enterococcal linear form plasmids (Fig. 5). The linear plasmids pELF_2008300 (106,518 bp) and pELF_2001564 (114,744 bp) share 96,190 bp at 99.99% identity. The unique 10,326 bp region in pELF_2008300 is flanked by IS1216 elements and carries several recombinases, transposases, and protein domains related to cell wall degradation. The main unique 17,708 bp region in pELF_2001564 carried a transposon with the vanA operon (Fig. 5). Upon querying NCBI for other highly similar sequences to pELF_2008300 and pELF_2001564, both scored the highest blastn identity, 100% for both, and highest coverage, 89% and 98%, respectively, with the linear plasmid pBA17124_P1, which carries optrA and was found in a clinical E. faecium isolated in 2019 from a patient in India (Fig. 5) (34). We then compared the plasmids in this study with the first described enterococcal linear form plasmids, pELF1, and pELF2 (33, 35) (Table S2). All four plasmids shared at least 75,950 bp, >97% identity, with one another including genes associated with DNA partitioning, transfer, and replication ftsK, soj, and repB, respectively. Although pELF_2008300 and pELF_2001564 uniquely carry optrA in a region that is inserted into radC, pELF2 has a copy of radC interrupted by a different antimicrobial resistance gene region. All three carry the antimicrobial resistance gene erm(A) in this region as well, whereas pELF1 does not carry radC or erm(A).
Fig 5.
Comparison of this study’s linear plasmids with optrA, the highly similar plasmid pBA17124_P1(34), and the first documented Enterococcal Linear Form plasmids, pELF1 and pELF2 (33, 35). Accession and plasmid length are shown under the plasmid name. Regions of similarity between sequences are connected by vertical blocks when there is a minimum of 500 bp and 80% BLASTN identity. Coding sequences are shown with arrows, and gene functions are color-coded as follows: vancomycin-related resistance genes are in orange, other resistance genes in red, radC in purple, DNA replication in brown, plasmid partitioning machinery in blue, parM in light blue, and IS elements or transposases in yellow.
To predict the distribution of pELF1-like plasmids in this data set, we queried short-read assemblies with in silico PCR using primers developed previously to identify the conserved terminal end sequences of pELF1-like plasmids (36). Among E. faecium isolates, 36/180 (20.0%) were predicted to harbor pELF1-like plasmid products, whereas no E. faecalis or E. lactis isolates contained predicted products. The isolates predicted to harbor pELF1-like plasmid products had 15 different STs within E. faecium and were found across seven different sites. Of all VRE isolates, 31/157 (19.7%) were predicted to harbor pELF1-like plasmid products, although this may be an underestimate due to primer-template variation and the potential for short-read assemblies to miss the end regions of these plasmids.
DISCUSSION
We characterized 404 enterococci from CDC’s 2018–2019 DHQP Sentinel Surveillance collection. Although all study isolates were submitted as E. faecalis or E. faecium as identified by clinical laboratories, the use of phylogenetic analysis revealed that 9.5% of submitted E. faecium isolates were E. lactis (formerly clade B E. faecium). MALDI-TOF MS and ANI analysis were not sufficient to differentiate E. faecium from E. lactis isolates. To date, custom in-house databases may be necessary to distinguish E. faecium from E. lactis isolates using MALDI-TOF MS (37). Recently, PCR assays have been designed to differentiate between E. faecium and E. lactis isolates (38). It is unknown how important differentiating these two species is for patient care.
Overall, resistance to vancomycin was more common among E. faecium isolates (78.3% VRE) than E. faecalis (7.8% VRE) or E. lactis (0% VRE), as previously demonstrated by others (18). The percent of E. faecium isolates showing resistance to vancomycin in this study was consistent with recent surveillance data examining AST profiles from bloodstream infections in the United States (2010–2019) (39) that found 72.5% of E. faecium were vancomycin-resistant. For all E. faecium isolates in our study, vanA was identified as the mechanism of vancomycin resistance, whereas Carvalhaes et al. identified vanA in 96.3% and vanB in 3.7% among vancomycin-resistant E. faecium isolates collected in the United States (39). The percent of vancomycin-resistant E. faecalis isolates was 7.8%, which is higher than the study by Carvalhaes et al. (39) that found that 3.6% of E. faecalis were resistant. All E. faecalis isolates in this study harbored vanA, compared with Carvalhaes et al. in which 85.5% of isolates contained vanA and 14.5% vanB (39). The elevated percent of vancomycin-resistant E. faecalis and lack of vanB seen in our surveillance may be explained by the variation in isolate source, as the Carvalhaes et al. study (39) focused on only isolates from bloodstream infections (39) and Sentinel Surveillance included isolates from bloodstream infections and from urine and other sources. In addition, the observation that vanA was the sole contributor to vancomycin resistance is not unexpected, as vanA has historically been reported as the predominant genotype in Canada, Europe, and the United States (40, 41).
Resistance to linezolid was rare in the 2018–2019 Sentinel Surveillance set, with 2% of E. faecalis isolates and 0.6% of E. faecium isolates showing resistance. This may represent a small increase in linezolid resistance among E. faecalis as previous DHQP Sentinel Surveillance from 2013 to 2016 showed 0.3% of E. faecalis and 0.6% of E. faecium isolates were linezolid-resistant, although the 2013–2016 surveillance period differed by two sites (5). Other US surveillance systems have reported E. faecalis and E. faecium linezolid resistance rates of 0.1% and 0.5%, respectively, among bloodstream infections in the United States from 2010 to 2019 (39). The frequency of daptomycin-resistant E. faecalis was 2% and E. faecium was 5.6% among the 2018–2019 Sentinel Surveillance set, compared with 0.5% and 5%, respectively, in the 2013–2016 DHQP Sentinel Surveillance set. This may represent a small increase in daptomycin resistance among E. faecalis. Other US surveillance systems have reported E. faecalis and E. faecium daptomycin resistance rates of 0% and 0.8%, respectively, among bloodstream infections in the United States from 2010 to 2019 (39). Continued surveillance is necessary to monitor resistance development to linezolid and daptomycin.
Overall, the diversity of genetic lineages in E. faecalis, E. faecium, and E. lactis was high, similar to previous surveillance of bloodstream infections caused by Enterococcus spp. (28). Vancomycin-resistant isolates had diverse genetic lineages even at single collection sites. This may indicate that we need to better understand the contribution of horizontal gene transfer to the spread of antimicrobial resistance among Enterococcus spp., as seen in several hospital-specific cases (35, 42).
Recently, strains of E. faecium that are nontypable due to the absence of the pstS MLST locus, referred to as pstS-null sequence types (e.g., ST1421, ST1424, and ST1478), have been reported to be rapidly spreading in Australia, the United Kingdom, Denmark, and Canada (20–23, 29). In addition, an increased proportion of daptomycin nonsusceptibility has been reported for ST1478 isolates (20). These pstS-null isolates were not a predominant ST among this set; however, they were identified in geographically diverse locations, and continued surveillance is necessary to monitor their distribution in the United States.
The mechanisms contributing to daptomycin resistance in enterococci have not been fully elucidated and resistance is associated with multiple pathways (11–13). Mutations in the LiaFSR system and the phospholipid metabolism enzyme, Cls, previously associated with daptomycin resistance (11, 12, 15, 30), were identified for only a subset of daptomycin-resistant E. faecalis and E. faecium isolates. These mutations were also detected among 18.8% of daptomycin-SDD E. faecium isolates, indicating the contribution of a variety of different resistance mechanisms.
Among the three E. faecium isolates that were not susceptible to linezolid, two isolates harbored optrA that were encoded on linear plasmids. This is the first description of the optrA gene on a linear plasmid in the United States. The optrA-encoding linear plasmids (designated pELF_2008300 and pELF_2001564) shared 99.9% sequence identity and were identified in two different E. faecium STs (ST78 and ST80), both of which are considered hospital-adapted sequence types associated with nosocomial infections worldwide (43). Both plasmids further shared 100% sequence identity and 89%–98% sequence coverage with a plasmid found in a clinical E. faecium isolated in 2019 from India (34), demonstrating the potential global spread of optrA on a linear plasmid (44).
Notably, one of the optrA-carrying linear plasmids also harbored a vanA cassette, leading to vancomycin resistance. The convergence of these resistance genes on the same genetic element/linear plasmid is concerning given that plasmids belonging to the pELF family have been shown to spread between Enterococcus spp. during healthcare-associated outbreaks (35, 42). In addition, previous laboratory work has demonstrated self-transmissibility and a low fitness cost of these plasmids, which could further facilitate the spread of these elements along with the associated resistance genes (33, 36, 45). Although studies indicate that linear enterococcal plasmids harboring vancomycin resistance genes may have only recently emerged, reports of linear plasmids harboring vanA, alone or in combination with other AR genes, have been reported elsewhere (33, 34, 36, 46).
Although bacterial linear plasmids were first described in the late 70s (47), the first report of a linear enterococcal plasmid was not until 2019 (33). Since then, linear plasmids in enterococci have been reported from multiple countries (33, 34, 36, 46); surveillance of VRE in Japan identified 3.4% of VRE that harbored pELF1-like plasmids, detected exclusively in E. faecium (36). In the present study, 19.7% of VRE were predicted to contain pELF1-like plasmid sequences similarly detected exclusively in E. faecium, indicating a relatively broad dissemination of pELF1-like plasmids among US E. faecium clinical isolates. Increased surveillance is necessary to better understand the distribution of these linear plasmids, their antimicrobial resistance gene content, and their clinical relevance/how they affect patient outcomes.
This work has limitations. First, the study design is based on a convenience sample rather than a random sample with limited metadata collected and no clinical outcome data. Thus, we cannot account for unknown biases that may have occurred. In addition, isolates were collected from nine sites and may not be representative of the true diversity at that site or in the United States. However, this study provides both reference AST and WGS data for more than 400 isolates collected from diverse sources.
E. faecium isolates showed higher levels of resistance to more antibiotics than E. faecalis, and resistance to linezolid and daptomycin was rare among the DHQP’s 2018–2019 Sentinel Surveillance collection. Whole genome sequencing revealed diverse STs among E. faecalis and E. faecium and the presence of optrA, associated with acquired linezolid resistance, encoded on a chromosomal transposon and on linear plasmids containing multiple antimicrobial resistance genes, including the vanA gene mediating vancomycin resistance. These data provide a snapshot of the AST profiles and molecular epidemiology of enterococci, important healthcare-associated pathogens. In the US, ongoing surveillance is necessary to detect possible future changes in AST patterns and/or monitor for increases in the mechanisms and emergence of new mechanisms of resistance.
MATERIALS AND METHODS
Bacterial isolates and identification
DHQP Sentinel Surveillance isolates are clinical isolates representing a convenience sample of bacteria collected annually from hospitalized patients from nine facilities in geographically distinct locations across the United States, including sites in California, Iowa, Maryland, Minnesota, New Mexico, New York, North Carolina, Pennsylvania, and Washington. Isolates are collected systematically at each site by choosing the first consecutive isolates of each organism. Isolates were cultured on BD BBL Trypticase soy agar II with 5% sheep blood (SBA) (Thermo Fisher Scientific, Waltham, MA, United States). Species identification was confirmed using MALDI-TOF mass spectrometry using a MALDI Biotyper, version 3.1 (Bruker Daltronics, Bremen, Germany).
Antimicrobial susceptibility testing
Broth microdilution (BMD) AST testing was performed on all isolates following the CLSI guidelines (48). BMD panels were prepared in-house consisting of 14 antibiotics at varying 2-fold serial dilutions in sterile 96-well microtiter plates (Caplugs, Rancho Domingues, CA, United States) and stored at −70°C until use. Each well contained 100 µL cation-adjusted Mueller-Hinton Broth (BD Difco, Sparks, MD, United States), and for daptomycin, there was a supplement of 50 µg/mL calcium chloride. See Table 2 for a complete list of antibiotics tested. Enterococcus faecalis ATCC 29212 and Staphylococcus aureus ATCC 29213 were used as quality control strains. Samples were streaked to purity on SBA. Colonies were resuspended in 5 mL REMEL 0.85% sterile saline (Thermo Fisher Scientific, Waltham, MA, United States) to a turbidity equivalent to a 0.5 MacFarland standard. Panels were inoculated with a 95-pin sterile inoculator (10 µL pickup; Caplugs, Buffalo, NY, United States), incubated at 35°C, then read at 16–20 hours for all antibiotics; vancomycin and gentamicin were also read at 24 hours; and streptomycin was also read at 24 and 48 hours. Results were interpreted according to CLSI guidelines (49).
Whole genome sequencing and analysis
All isolates underwent whole genome short-read sequencing (Illumina MiSeq or NovaSeq, San Diego, CA). Genomic DNA was extracted from colonies cultured overnight on SBA using the Promega Maxwell 16 Cell Low Elution Volume DNA Purification Kit and Maxwell 16 MDx Instrument (Madison, WI, United States). Sequencing using the MiSeq was performed as previously described (5). For NovaSeq WGS, genomic DNA was sheared to a mean size of 600 bp using a Covaris LE220 focused ultrasonicator (Covaris Inc., Woburn, MA). DNA fragments were AMPure XP bead cleaned (Beckman Coulter Inc., Indianapolis, IN) and used to prepare dual-indexed sequencing libraries using NEBNext Ultra library prep reagents (New England Biolabs Inc., Ipswich, MA) and barcoding indices synthesized in the CDC Biotechnology Core Facility. Libraries were analyzed for size and concentration, normalized, pooled, and denatured for loading onto flow cells for cluster generation. Sequencing was performed using the MiSeq Reagent Kit v2 (500 cycles) and Illumina MiSeq System (San Diego, CA) or on a NovaSeq using the NovaSeq 6000 Reagent Kit (500 cycles). On completion, sequence reads were filtered for read quality, base called, and demultiplexed using bcl2fastq (v2.19). Short-read sequences (MiSeq and NovaSeq) were analyzed using the QuAISAR-H pipeline; a description of publicly available tools and versions can be found on the QuAISAR-H repository (https://github.com/DHQP/QuAISAR_singularity). Specifically, raw short-read sequences were processed with BBDuk v38.90 (https://sourceforge.net/projects/bbmap/) and fastp v.0.20.1 (50) to remove adapters and PhiX then quality trim sequences. ANI was computed using pyANI v 0.2.10 (51) against NCBI’s RefSeq database. Trimmed sequences were used for high-quality single-nucleotide variant (hqSNV) analyses and hybrid genome assembly.
Antimicrobial resistance genes and point mutations were identified using GAMMA (52) against a manually curated non-redundant database comprising antimicrobial resistance genes from ResFinder (53), ARG-ANNOT (54), and NCBI’s AMRFinderPlus (55) (all accessed 06/06/2024); minimum of 98% sequence identity and 90% sequence coverage was used. Mutations associated with linezolid resistance within the 23S rRNA genes from E. faecium and E. faecalis were determined by mapping short-read sequences to a linezolid-susceptible reference using Bowtie2 (56), processed using SamTools (57), then hqSNV-called using VarScan v2.4.1 (58) using mpileup2cns with the parameters: --min-coverage 8 --min-reads2 2 --min-var-freq 0.01. Potential pELF1-like linear plasmids were identified in the short-read assembled genomes using ipcress with default settings for in silico PCR with previously published primers targeting the ends of pELF1(36).Table S1
Phylogenetic and MLST analyses
Short-read assembled genomes were annotated with Prokka v1.14.3 (59), and a core genome alignment was created using Roary v3.12.0 and MAFFT v7.407 with default parameters. A maximum likelihood tree was created on the core genome alignment using RAxML v8.2.12 with model GTRCAT and 100 bootstraps. Aus0004 and 1–231-501, were used as references to identify E. faecium, COM15, was used as the reference for E. lactis and ATCC19433 as a reference for E. faecalis. The diversity of E. faecalis, E. faecium, and E. lactis MLSTs was assessed using the Simpson’s Diversity Index with R’s vegan package. Global sequence data for the pstS-null analysis were obtained from NCBI’s SRA and GenBank databases pulled from the following BioProjects: PRJEB13012, PRJNA816868, and PRJEB25215. Assemblies were pulled from NCBI unless they were not available, in which case sequence data were pulled from NCBI and assembled as described before. Then, the MLST was identified to separate based on ST and core-gene based maximum likelihood phylogenetic trees for ST1421 and ST1478 were generated as described before. Core SNPs were identified using snp-dists (https://github.com/tseemann/snp-dists) on the core gene alignment generated by Roary.
Identification of linear plasmids by S1 nuclease pulsed-field gel electrophoresis
Isolates identified as non-susceptible (intermediate or resistant) to linezolid (MIC >4 µg/mL) were first analyzed for the presence of optrA using PCR following the previously described procedure (5). PCR samples that were optrA-positive were further analyzed by S1 nuclease pulsed-field gel electrophoresis (PFGE) as described previously (5). Briefly, samples were cultured to purity on SBA. Cells were embedded in 1.5% SeaKem Gold agarose (Thermo Fisher Scientific, Waltham, MA, United States) and lysed in Gram-positive lysis buffer (0.05% Tris, 0.1% EDTA, 0.01% N-lauroylsarcosine sodium salt). Agarose plug slices were cut and digested using 20U S1 nuclease (Takara Bio, Mountain View, CA, United States). Samples were then subjected to PFGE at 6 V/cm, 14°C for 20 hr with switch times of 1 and 25 s.
Long-read analyses
Isolates identified as carrying optrA were selected for long-read sequencing [Oxford Nanopore Technologies (ONT) MinION, Oxford, UK]. High molecular weight (HMW) genomic DNA was extracted using the Qiagen QIAamp DNA Blood Mini Kit (Germantown, MD), quantified using the Qubit dsDNA Broad Range Assay Kit (ThermoFisher Scientific, Waltham, MA), and purity was confirmed (ThermoFisher Scientific Nanodrop 2000 Spectrophotometer). The genomic library was prepared using 400 ng of HMW genomic DNA and the ONT Rapid Barcoding Sequencing Kit (SQK-RBK004), followed by sequencing on the R9.4.1 flow cell; the optional AMPure XP bead purification step was included during library prep. Guppy v.3.0.7 was used to live basecall long-read sequences (ONT MinION) using the fast basecalling model, excluding poor-quality reads (quality score <Q7). Porechop v.0.2.3 (https://github.com/rrwick/Porechop) was used to demultiplex and trim adapters, then Filtlong v0.2.0 (https://github.com/rrwick/Filtlong) was used to additionally filter out poor quality reads with parameters --min_length 1000 --keep_percent 90 --target_bases 5000000000 --trim --split 500. Using quality-controlled short- and long-read sequences, hybrid genome assemblies were generated for each sample using Unicycler v.0.4.8 (60) with default parameters. ContigExtender v0.1 (61) with default parameters was used to extend the linear plasmid pELF_2001564. Known linear form enterococcal plasmids were used for comparison (Table S2). To compare plasmids, assemblies were annotated with the Prokaryotic Genome Annotation Pipeline (62), and then visualized with BLASTN and Easyfig (63).
ACKNOWLEDGMENTS
The authors wish to thank the staff of all participating clinical microbiology laboratories. Without their collection and submission of isolates, this work would not have been possible. The authors would also like to thank Erisa Sula for her contribution to this work.
This work was made possible, in part, through the CDC’s Safety and Healthcare Epidemiology Prevention Research Development (SHEPheRD) Program funding awarded to J.K.J. (contract 200-2016-91793).
Contributor Information
Amy S. Gargis, Email: AGargis@cdc.gov.
Benjamin P. Howden, The Peter Doherty Institute for Infection and Immunity, Melbourne, Australia
DATA AVAILABILITY
Raw sequencing reads and assembled genomes were deposited in NCBI GenBank under PRJNA573902; accessions are listed in Table S1.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/aac.00591-24.
Figures S1 and S2; Table S2.
Characteristics of isolates, including public accessions, taxonomic identification, site, source, identified resistance mechanisms, and antimicrobial susceptibility testing.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. CDC . 2019. Antibiotic resistance threats in the United States, 2019. Atlanta, GA: Department of Health and Human Services C, Department of Health and Human Services, CDC [Google Scholar]
- 2. Miller WR, Murray BE, Rice LB, Arias CA. 2016. Vancomycin-resistant enterococci: therapeutic challenges in the 21st century. Infect Dis Clin North Am 30:415–439. doi: 10.1016/j.idc.2016.02.006 [DOI] [PubMed] [Google Scholar]
- 3. Jernigan JA, Hatfield KM, Wolford H, Nelson RE, Olubajo B, Reddy SC, McCarthy N, Paul P, McDonald LC, Kallen A, Fiore A, Craig M, Baggs J. 2020. Multidrug-resistant bacterial infections in U.S. hospitalized patients, 2012-2017. N Engl J Med 382:1309–1319. doi: 10.1056/NEJMoa1914433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. CDC . 2022. COVID-19: U.S. impact on antimicrobial resistance, special report 2022. doi: 10.15620/cdc:117915 [DOI]
- 5. Gargis AS, Spicer LM, Kent AG, Zhu W, Campbell D, McAllister G, Ewing TO, Albrecht V, Stevens VA, Sheth M, Padilla J, Batra D, Johnson JK, Halpin AL, Rasheed JK, Elkins CA, Karlsson M, Lutgring JD. 2021. Sentinel surveillance reveals emerging daptomycin-resistant ST736 Enterococcus faecium and multiple mechanisms of linezolid resistance in enterococci in the United States. Front Microbiol 12:807398. doi: 10.3389/fmicb.2021.807398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. García-Solache M, Rice LB. 2019. The enterococcus: a model of adaptability to its environment. Clin Microbiol Rev 32:e00058-18. doi: 10.1128/CMR.00058-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hasman H, Clausen PTLC, Kaya H, Hansen F, Knudsen JD, Wang M, Holzknecht BJ, Samulioniené J, Røder BL, Frimodt-Møller N, Lund O, Hammerum AM. 2019. LRE-Finder, a Web tool for detection of the 23S rRNA mutations and the optrA, cfr, cfr(B) and poxtA genes encoding linezolid resistance in enterococci from whole-genome sequences. J Antimicrob Chemother 74:1473–1476. doi: 10.1093/jac/dkz092 [DOI] [PubMed] [Google Scholar]
- 8. Sadowy E. 2018. Linezolid resistance genes and genetic elements enhancing their dissemination in enterococci and streptococci. Plasmid 99:89–98. doi: 10.1016/j.plasmid.2018.09.011 [DOI] [PubMed] [Google Scholar]
- 9. Bi R, Qin T, Fan W, Ma P, Gu B. 2018. The emerging problem of linezolid-resistant enterococci. J Glob Antimicrob Resist 13:11–19. doi: 10.1016/j.jgar.2017.10.018 [DOI] [PubMed] [Google Scholar]
- 10. Prater AG, Mehta HH, Kosgei AJ, Miller WR, Tran TT, Arias CA, Shamoo Y. 2019. Environment shapes the accessible daptomycin resistance mechanisms in Enterococcus faecium. Antimicrob Agents Chemother 63:e00790-19. doi: 10.1128/AAC.00790-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prater AG, Mehta HH, Beabout K, Supandy A, Miller WR, Tran TT, Arias CA, Shamoo Y. 2021. Daptomycin resistance in Enterococcus faecium can be delayed by disruption of the LiaFSR stress response pathway. Antimicrob Agents Chemother 65:e01317-20. doi: 10.1128/AAC.01317-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Diaz L, Tran TT, Munita JM, Miller WR, Rincon S, Carvajal LP, Wollam A, Reyes J, Panesso D, Rojas NL, Shamoo Y, Murray BE, Weinstock GM, Arias CA. 2014. Whole-genome analyses of Enterococcus faecium isolates with diverse daptomycin MICs. Antimicrob Agents Chemother 58:4527–4534. doi: 10.1128/AAC.02686-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang G, Yu F, Lin H, Murugesan K, Huang W, Hoss AG, Dhand A, Lee LY, Zhuge J, Yin C, Montecalvo M, Dimitrova N, Fallon JT. 2018. Evolution and mutations predisposing to daptomycin resistance in vancomycin-resistant Enterococcus faecium ST736 strains. PLoS One 13:e0209785. doi: 10.1371/journal.pone.0209785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miller WR, Tran TT, Diaz L, Rios R, Khan A, Reyes J, Prater AG, Panesso D, Shamoo Y, Arias CA. 2019. LiaR-independent pathways to daptomycin resistance in Enterococcus faecalis reveal a multilayer defense against cell envelope antibiotics. Mol Microbiol 111:811–824. doi: 10.1111/mmi.14193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arias CA, Panesso D, McGrath DM, Qin X, Mojica MF, Miller C, Diaz L, Tran TT, Rincon S, Barbu EM, Reyes J, Roh JH, Lobos E, Sodergren E, Pasqualini R, Arap W, Quinn JP, Shamoo Y, Murray BE, Weinstock GM. 2011. Genetic basis for in vivo daptomycin resistance in enterococci. N Engl J Med 365:892–900. doi: 10.1056/NEJMoa1011138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bacteria FTFoCA-R . 2020. National action plan for combating antibiotic-resistant bacteria (CARB) 2020-2025
- 17. Carvalhaes CG, Sader HS, Streit JM, Castanheira M, Mendes RE. 2020. 1629. Vancomycin resistance in Enterococcus faecium clinical isolates responsible for bloodstream infections in US hospitals over ten years (2010-2019) and activity of oritavancin. Open Forum Infect Dis 7:S806–S807. doi: 10.1093/ofid/ofaa439.1809 [DOI] [Google Scholar]
- 18. Mendes RE, Castanheira M, Farrell DJ, Flamm RK, Sader HS, Jones RN. 2016. Longitudinal (2001-14) analysis of enterococci and VRE causing invasive infections in European and US hospitals, including a contemporary (2010-13) analysis of oritavancin in vitro potency. J Antimicrob Chemother 71:3453–3458. doi: 10.1093/jac/dkw319 [DOI] [PubMed] [Google Scholar]
- 19. Pfaller MA, Cormican M, Flamm RK, Mendes RE, Jones RN. 2019. Temporal and geographic variation in antimicrobial susceptibility and resistance patterns of enterococci: results from the SENTRY antimicrobial surveillance program, 1997-2016. Open Forum Infect Dis 6:S54–S62. doi: 10.1093/ofid/ofy344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McCracken M, Mitchell R, Smith S, Hota S, Conly J, Du T, Embil J, Johnston L, Ormiston D, Parsonage J, Simor A, Wong A, Golding G, Canadian Nosocomial Infection Surveillance P. 2020. Emergence of pstS-null vancomycin-resistant Enterococcus faecium clone ST1478, Canada, 2013-2018. Emerg Infect Dis 26:2247–2250. doi: 10.3201/eid2609.201576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lemonidis K, Salih TS, Dancer SJ, Hunter IS, Tucker NP. 2019. Emergence of an Australian-like pstS-null vancomycin resistant Enterococcus faecium clone in Scotland. PLoS One 14:e0218185. doi: 10.1371/journal.pone.0218185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hammerum AM, Justesen US, Pinholt M, Roer L, Kaya H, Worning P, Nygaard S, Kemp M, Clausen ME, Nielsen KL, Samulioniené J, Kjærsgaard M, Østergaard C, Coia J, Søndergaard TS, Gaini S, Schønning K, Westh H, Hasman H, Holzknecht BJ. 2019. Surveillance of vancomycin-resistant enterococci reveals shift in dominating clones and national spread of a vancomycin-variable vanA Enterococcus faecium ST1421-CT1134 clone, Denmark, 2015 to March 2019. Euro Surveill 24:1900503. doi: 10.2807/1560-7917.ES.2019.24.34.1900503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kleinman DR, Mitchell R, McCracken M, Hota SS, Golding GR, Group CVW, Smith SW. 2023. Vancomycin-resistant Enterococcus sequence type 1478 spread across hospitals participating in the Canadian nosocomial infection surveillance program from 2013 to 2018. Infect Control Hosp Epidemiol 44:17–23. doi: 10.1017/ice.2022.7 [DOI] [PubMed] [Google Scholar]
- 24. Belloso Daza MV, Cortimiglia C, Bassi D, Cocconcelli PS. 2021. Genome-based studies indicate that the Enterococcus faecium Clade B strains belong to Enterococcus lactis species and lack of the hospital infection associated markers. Int J Syst Evol Microbiol 71:004948. doi: 10.1099/ijsem.0.004948 [DOI] [PubMed] [Google Scholar]
- 25. Satlin MJ, Nicolau DP, Humphries RM, Kuti JL, Campeau SA, Lewis Ii JS, Weinstein MP, Jorgensen JH. 2020. Development of daptomycin susceptibility breakpoints for Enterococcus faecium and revision of the breakpoints for other enterococcal species by the clinical and laboratory standards institute. Clin Infect Dis 70:1240–1246. doi: 10.1093/cid/ciz845 [DOI] [PubMed] [Google Scholar]
- 26. Lam MMC, Seemann T, Bulach DM, Gladman SL, Chen H, Haring V, Moore RJ, Ballard S, Grayson ML, Johnson PDR, Howden BP, Stinear TP. 2012. Comparative analysis of the first complete Enterococcus faecium genome. J Bacteriol 194:2334–2341. doi: 10.1128/JB.00259-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Palmer KL, Carniol K, Manson JM, Heiman D, Shea T, Young S, Zeng Q, Gevers D, Feldgarden M, Birren B, Gilmore MS. 2010. High-quality draft genome sequences of 28 Enterococcus sp. isolates. J Bacteriol 192:2469–2470. doi: 10.1128/JB.00153-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Contreras GA, Munita JM, Simar S, Luterbach C, Dinh AQ, Rydell K, Sahasrabhojane PV, Rios R, Diaz L, Reyes K, et al. 2022. Contemporary clinical and molecular epidemiology of vancomycin-resistant enterococcal bacteremia: a prospective multicenter cohort study (VENOUS I). Open Forum Infect Dis 9:fab616. doi: 10.1093/ofid/ofab616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carter GP, Buultjens AH, Ballard SA, Baines SL, Tomita T, Strachan J, Johnson PDR, Ferguson JK, Seemann T, Stinear TP, Howden BP. 2016. Emergence of endemic MLST non-typeable vancomycin-resistant Enterococcus faecium. J Antimicrob Chemother 71:3367–3371. doi: 10.1093/jac/dkw314 [DOI] [PubMed] [Google Scholar]
- 30. Miller C, Kong J, Tran TT, Arias CA, Saxer G, Shamoo Y. 2013. Adaptation of Enterococcus faecalis to daptomycin reveals an ordered progression to resistance. Antimicrob Agents Chemother 57:5373–5383. doi: 10.1128/AAC.01473-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li D, Li X-Y, Schwarz S, Yang M, Zhang S-M, Hao W, Du X-D. 2019. Tn6674 is a novel enterococcal optrA-carrying multiresistance transposon of the Tn554 family. Antimicrob Agents Chemother 63:e00809-19. doi: 10.1128/AAC.00809-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cornell CR, Marasini D, Fakhr MK. 2018. Molecular characterization of plasmids harbored by actinomycetes isolated from the great salt plains of Oklahoma using PFGE and next generation whole genome sequencing. Front Microbiol 9:2282. doi: 10.3389/fmicb.2018.02282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hashimoto Y, Taniguchi M, Uesaka K, Nomura T, Hirakawa H, Tanimoto K, Tamai K, Ruan G, Zheng B, Tomita H. 2019. Novel multidrug-resistant enterococcal mobile linear plasmid pELF1 encoding vanA and vanM gene clusters from a Japanese vancomycin-resistant enterococci isolate. Front Microbiol 10:2568. doi: 10.3389/fmicb.2019.02568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bakthavatchalam YD, Puraswani M, Livingston A, Priya M, Venkatesan D, Sharma D, Iyadurai R, Pichamuthu K, Veeraraghavan B, Mathur P. 2022. Novel linear plasmids carrying vanA cluster drives the spread of vancomycin resistance in Enterococcus faecium in India. J Glob Antimicrob Resist 29:168–172. doi: 10.1016/j.jgar.2022.03.013 [DOI] [PubMed] [Google Scholar]
- 35. Hashimoto Y, Kita I, Suzuki M, Hirakawa H, Ohtaki H, Tomita H. 2020. First report of the local spread of vancomycin-resistant enterococci ascribed to the interspecies transmission of a vanA gene cluster-carrying linear plasmid. mSphere 5:e00102-20. doi: 10.1128/mSphere.00102-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hashimoto Y, Suzuki M, Kobayashi S, Hirahara Y, Kurushima J, Hirakawa H, Nomura T, Tanimoto K, Tomita H. 2023. Enterococcal linear plasmids adapt to Enterococcus faecium and spread within multidrug-resistant clades. Antimicrob Agents Chemother 67:e0161922. doi: 10.1128/aac.01619-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim E, Yang SM, Kim HJ, Kim HY. 2022. Differentiating between Enterococcus faecium and Enterococcus lactis by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Foods 11:1046. doi: 10.3390/foods11071046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Belloso Daza MV, Almeida-Santos AC, Novais C, Read A, Alves V, Cocconcelli PS, Freitas AR, Peixe L. 2022. Distinction between Enterococcus faecium and Enterococcus lactis by a gluP PCR-based assay for accurate identification and diagnostics. Microbiol Spectr 10:e0326822. doi: 10.1128/spectrum.03268-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carvalhaes CG, Sader HS, Streit JM, Castanheira M, Mendes RE. 2022. Activity of oritavancin against Gram-positive pathogens causing bloodstream infections in the United States over 10 years: focus on drug-resistant enterococcal subsets (2010-2019). Antimicrob Agents Chemother 66:e0166721. doi: 10.1128/AAC.01667-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Simner PJ, Adam H, Baxter M, McCracken M, Golding G, Karlowsky JA, Nichol K, Lagacé-Wiens P, Gilmour MW, Hoban DJ, Zhanel GG, Canadian Antimicrobial Resistance Alliance (CARA) . 2015. Epidemiology of vancomycin-resistant enterococci in Canadian hospitals (CANWARD study, 2007 to 2013). Antimicrob Agents Chemother 59:4315–4317. doi: 10.1128/AAC.00384-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Deshpande LM, Fritsche TR, Moet GJ, Biedenbach DJ, Jones RN. 2007. Antimicrobial resistance and molecular epidemiology of vancomycin-resistant enterococci from North America and Europe: a report from the SENTRY antimicrobial surveillance program. Diagn Microbiol Infect Dis 58:163–170. doi: 10.1016/j.diagmicrobio.2006.12.022 [DOI] [PubMed] [Google Scholar]
- 42. Fujiya Y, Harada T, Sugawara Y, Akeda Y, Yasuda M, Masumi A, Hayashi J, Tanimura N, Tsujimoto Y, Shibata W, Yamaguchi T, Kawahara R, Nishi I, Hamada S, Tomono K, Kakeya H. 2021. Transmission dynamics of a linear vanA-plasmid during a nosocomial multiclonal outbreak of vancomycin-resistant enterococci in a non-endemic area, Japan. Sci Rep 11:14780. doi: 10.1038/s41598-021-94213-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Monteiro Marques J, Coelho M, Santana AR, Pinto D, Semedo-Lemsaddek T. 2023. Dissemination of enterococcal genetic lineages: a one health perspective. Antibiotics (Basel) 12:1140. doi: 10.3390/antibiotics12071140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bakthavatchalam YD, Vasudevan K, Babu P, Neeravi AR, Narasiman V, Veeraraghavan B. 2021. Genomic insights of optrA-carrying linezolid-resistant Enterococcus faecium using hybrid assembly: first report from India. J Glob Antimicrob Resist 25:331–336. doi: 10.1016/j.jgar.2021.04.005 [DOI] [PubMed] [Google Scholar]
- 45. Boumasmoud M, Dengler Haunreiter V, Schweizer TA, Meyer L, Chakrakodi B, Schreiber PW, Seidl K, Kühnert D, Kouyos RD, Zinkernagel AS. 2022. Genomic surveillance of vancomycin-resistant Enterococcus faecium reveals spread of a linear plasmid conferring a nutrient utilization advantage. mBio 13:e0377121. doi: 10.1128/mbio.03771-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Egan SA, Kavanagh NL, Shore AC, Mollerup S, Samaniego Castruita JA, O’Connell B, McManus BA, Brennan GI, Pinholt M, Westh H, Coleman DC. 2022. Genomic analysis of 600 vancomycin-resistant Enterococcus faecium reveals a high prevalence of ST80 and spread of similar vanA regions via IS1216E and plasmid transfer in diverse genetic lineages in Ireland. J Antimicrob Chemother 77:320–330. doi: 10.1093/jac/dkab393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hinnebusch J, Tilly K. 1993. Linear plasmids and chromosomes in bacteria. Mol Microbiol 10:917–922. doi: 10.1111/j.1365-2958.1993.tb00963.x [DOI] [PubMed] [Google Scholar]
- 48. CLSI . 2018. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard. In CLSI standard M07, 11th ed. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 49. CLSI . 2021. Performance standards for antimicrobial susceptibility testing CLSI supplement M100. 31st ed. Clinical and Laboratory Standards Institute, Wayne, PA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen S, Zhou Y, Chen Y, Gu J. 2018. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34:i884–i890. doi: 10.1093/bioinformatics/bty560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pritchard L, Glover RH, Humphris S, Elphinstone JG, Toth IK. 2016. Genomics and taxonomy in diagnostics for food security: soft-rotting enterobacterial plant pathogens. Anal Methods 8:12–24. doi: 10.1039/C5AY02550H [DOI] [Google Scholar]
- 52. Stanton RA, Vlachos N, Halpin AL. 2022. GAMMA: a tool for the rapid identification, classification and annotation of translated gene matches from sequencing data. Bioinformatics 38:546–548. doi: 10.1093/bioinformatics/btab607 [DOI] [PubMed] [Google Scholar]
- 53. Zankari E. 2014. Comparison of the web tools ARG-ANNOT and ResFinder for detection of resistance genes in bacteria. Antimicrob Agents Chemother 58:4986. doi: 10.1128/AAC.02620-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gupta SK, Padmanabhan BR, Diene SM, Lopez-Rojas R, Kempf M, Landraud L, Rolain JM. 2014. ARG-ANNOT, a new bioinformatic tool to discover antibiotic resistance genes in bacterial genomes. Antimicrob Agents Chemother 58:212–220. doi: 10.1128/AAC.01310-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Feldgarden M, Brover V, Haft DH, Prasad AB, Slotta DJ, Tolstoy I, Tyson GH, Zhao S, Hsu CH, McDermott PF, Tadesse DA, Morales C, Simmons M, Tillman G, Wasilenko J, Folster JP, Klimke W. 2019. Validating the AMRFinder tool and resistance gene database by using antimicrobial resistance genotype-phenotype correlations in a collection of isolates. Antimicrob Agents Chemother 63:e00483-19. doi: 10.1128/AAC.00483-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Langdon WB. 2015. Performance of genetic programming optimised Bowtie2 on genome comparison and analytic testing (GCAT) benchmarks. BioData Min 8:1. doi: 10.1186/s13040-014-0034-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup . 2009. The sequence Alignment/Map format and SAMtools. Bioinformatics 25:2078–2079. doi: 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Koboldt DC, Zhang Q, Larson DE, Shen D, McLellan MD, Lin L, Miller CA, Mardis ER, Ding L, Wilson RK. 2012. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res 22:568–576. doi: 10.1101/gr.129684.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. doi: 10.1093/bioinformatics/btu153 [DOI] [PubMed] [Google Scholar]
- 60. Wick RR, Judd LM, Gorrie CL, Holt KE. 2017. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol 13:e1005595. doi: 10.1371/journal.pcbi.1005595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Deng Z, Delwart E. 2021. ContigExtender: a new approach to improving de novo sequence assembly for viral metagenomics data. BMC Bioinformatics 22:119. doi: 10.1186/s12859-021-04038-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tatusova T, DiCuccio M, Badretdin A, Chetvernin V, Nawrocki EP, Zaslavsky L, Lomsadze A, Pruitt KD, Borodovsky M, Ostell J. 2016. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res 44:6614–6624. doi: 10.1093/nar/gkw569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sullivan MJ, Petty NK, Beatson SA. 2011. Easyfig: a genome comparison visualizer. Bioinformatics 27:1009–1010. doi: 10.1093/bioinformatics/btr039 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1 and S2; Table S2.
Characteristics of isolates, including public accessions, taxonomic identification, site, source, identified resistance mechanisms, and antimicrobial susceptibility testing.
Data Availability Statement
Raw sequencing reads and assembled genomes were deposited in NCBI GenBank under PRJNA573902; accessions are listed in Table S1.