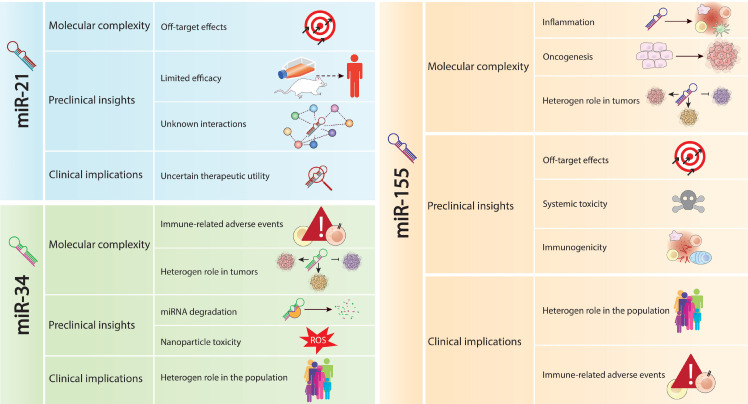
Figure 5.
Challenges associated with the withdrawal of miRNAs from clinical trials. This figure outlines the main challenges leading to the withdrawal of miRNAs, including miR-21, miR-34a, and miR-155, from clinical trials. The challenges include off-target effects, limited efficacy in preclinical models, inadequate understanding of regulatory networks, and the focus on biomarker potential rather than therapeutic utility for miR-21. For miR-34a, challenges involve immune-related adverse events, preclinical translation hurdles, molecular complexity, and survival rates. miR-155's challenges encompass molecular complexity, clinical implications, and difficulties in translating promising preclinical data into clinical practice. This comprehensive analysis underscores the multifaceted challenges encountered in miRNA-based therapies, impacting their clinical translation and therapeutic efficacy.