Abstract
Background:
Simulation for training is becoming a trend topic worldwide, even if its applications are commonly limited to adulthood. Ultrasound-guided procedures require practice and experience—especially in the pediatric field, where the small size of the involved anatomical structures poses major problems. In this context, a realistic 3D printed pediatric phantom for training of the ultrasound-guided placement of peripheral central venous catheters in children was developed.
Materials and methods:
Starting from Computed Tomography scans of an 8 years-old girl, her left arm was virtually reconstructed—including bones, arteries, and veins—through a semi-automatic segmentation process. According to preliminary results, the most suitable 3D printing technologies to reproduce the different anatomical structures of interest were selected, considering both direct and indirect 3D printing techniques. Experienced operators were asked to evaluate the efficacy of the final model through a dedicated questionnaire.
Results:
Vessels produced through indirect 3D printing latex dipping technique exhibited the best echogenicity, thickness, and mechanical properties to mimic real children’s venous vessels, while arteries—not treated and/or punctured during the procedure—were directly 3D printed through Material Jetting technology. An external mold—mimicking the arm skin—was 3D printed and a silicone-based mixture was poured to reproduce real patient’s soft tissues. Twenty expert specialists were asked to perform the final model’s validation. The phantom was rated as highly realistic in terms of morphology and functionality for the overall simulation, especially for what concerns vessels and soft tissues’ response to puncturing. On the other hand, the involved structures’ US appearance showed the lower score.
Conclusions:
The present work shows the feasibility of a patient-specific 3D printed phantom for simulation and training in pediatric ultrasound-guided procedures.
Keywords: 3D printing, 3D printed pediatric phantom, cardiovascular access, surgical simulation, ultrasound-guided procedure
Introduction
Ultrasound (US) guided placement of venous peripherally inserted central catheter (PICC) in children requires practice and experience. A successful venipuncture can be achieved only after repeated puncturing, due to the small size of the anatomical structures. As the caliber of the vessel decreases, the technical difficulty in cannulation increases. 1 Repeated attempts at venipuncture are associated with greater local trauma, intramural venous hematomas, and other local alterations that can lead to an increased risk of venous thrombosis, even making the procedure ineffective. 2
Today, the medical and surgical fields are knowing a rising interest in the development of simulation and training devices, which allows trainees to practice, make mistakes, reflect, and question themselves about possible failures, with no risk for the patients’ safety.
To date, the PICC positioning training involves the use of animal models (generally turkey meat) or agar-agar pads with silicon tubes to represent arterial and venous vessels; while being a low-cost solution, such models have some major limitations in terms of level of realism, repeatability, and life span of the model itself.
In this context, additive manufacturing technology, mostly known as 3D printing (3DP), is emerging as a promising solution for the creation of realistic replica of patient-specific anatomies. Thanks to the ability of producing extremely complex geometries and to the lower production cost for a single component, with respect to traditional technologies, 3DP perfectly fits the needs of patient-specific anatomical modeling. In fact, this application involves the production of complex geometries—as anatomical structures are—and commonly a single or few copies of a model are required: the success of 3DP technology in medical field is witnessed by the exponential increase in the amount of published literature over the past few years in several clinical-surgical disciplines.3 –6
The purpose of the present study is to develop a 3D printed pediatric arm phantom with realistic echogenicity, morphological and mechanical properties to be used for training in the US-guided positioning of PICC in children.
Materials and methods
The pediatric arm phantom must fulfill the following main requirements:
Morphology: realistic size of all the anatomical structures
Echogenicity: real-like US visibility of the different anatomical structures
Mechanical properties: reproduction of the compressibility of vessels and soft tissues, to get a real-like behavior of the phantom during the execution of an US-guided puncture of the venous vessel.
The phantom was designed and manufactured through three main development stages, described in the following.
Stage 1: Virtual model generation
The first step was the selection of suitable radiological images. From the Hospital’s Picture Archiving and Communication System (PACS), a full body Computed Tomography (CT) with 0.5 mm slice thickness performed on an 8 years-old girl was selected; the CT scan included the entire left upper limb, acquired with a multilayer spiral technique before and after the intravenous administration of iodized contrast medium (Iodixanol 320). Arterial and venous CT phases allowed the proper visibility of all the anatomical structures to be included in the phantom, namely:
homerus, reconstructed from the venous CT phase;
brachial artery, reconstructed from the arterial CT phase;
brachial, basilica, and cephalic veins, reconstructed from the venous CT phase;
soft tissues (including muscles and subcutaneous fat), reconstructed from the arterial CT phase.
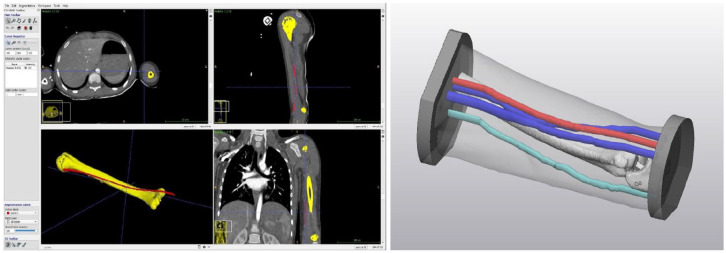
The image segmentation process on the selected CT scans was performed through a semi-automatic algorithm in the open-source software ITK-SNAP (http://www.itksnap.org/), 7 identifying and isolating all the different structures of interest relying on their gray levels and a series of other morphological parameters. The segmentation resulted in a series of 3D virtual rendering—one for each structure of interest—which were converted in Standard Triangulation Language (STL) format, the most common file formats accepted as input for all the 3DP technologies.
The STL files were then imported into 3-Matic Medical tool (Materialise®) (https://www.materialise.com/en/healthcare/mimics-innovation-suite/3-matic), a certified professional software for the 3D virtual models’ processing and manipulation, to create additional support structures and to perform all the final model’s refinements (Figure 1).
Figure 1.
ITK-Snap segmentation performed on selected CT scans (left) and final 3D virtual pediatric arm model (right).
Stage 2: Manufacturing materials’ testing
Anatomical structures
Preliminary tests were performed to assess the most suitable materials able to reproduce bony structures and both arterial and venous vessels, analyzing the critical issues of each material in terms of morphological, mechanical, and echogenicity properties.
According to the available data, 8 the 3DP technologies and materials considered for our purposes were:
Material Jetting technology with an Objet260 Connex3—Stratasys® 3D printer; it offers the possibility to deploy and mix multiple photopolymer resins of various colors and with different degrees of deformability;
Binder Jetting technology with a ProJet 460 Plus—3DSystems®; it works with a chalk powder, selectively glued, and colored by two printheads.
For what concern the reproduction of bony structures—that is, the humerus—Binder Jetting technology was immediately selected thanks to prior knowledge; on the contrary, deeper investigation was required to select the most suitable 3DP materials for the reproduction of vascular structures. 8
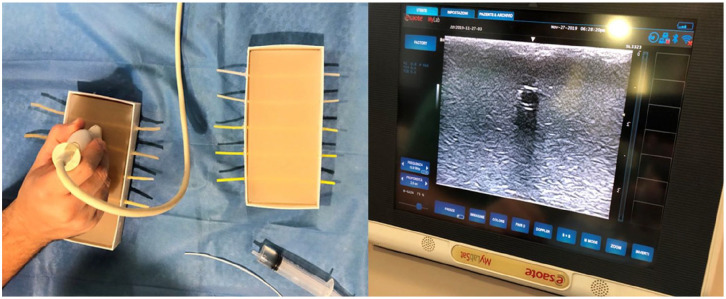
To test the materials’ US appearance, different 2.5 mm × 150 mm cylinder samples with wall thicknesses ranging from 0.5 to 1 mm—that is, with a caliber comparable to the one of blood vessels of school-age children—were produced with the different 3DP technologies under analysis and positioned in 180 mm × 90 mm × 30 mm molds filled with an Agar-based solution (Figure 2). To match the US-visibility of vessels structures, cylinder samples were filled with water at room temperature. Given the small caliper and the subtle wall thickness of the vessels of interest, direct 3DP resulted not suitable for the manufacturing. The resulting lumen was impossible to be filled with water, due to small residuals of support material, and to the delamination easily occuring on the subtle vessels wall during the cleaning. A dipping technique was then tested as an alternative: the vessel lumen was 3D printed and coated with multiple latex layers. Then, the inner core was removed leaving a hollow structure made only of latex, to resemble the vessel wall. This allowed for the creation of vessels with a thinner and more resistant wall, therefore suitable for the purposes of the study. The echogenicity and the puncture behavior of vessels made with a different number of latex layers was also tested.
Figure 2.
Preliminary echogenicity tests performed on different 3DP materials and latex vessels submerged in agar-agar mixture.
Preliminary tests showed a good realism of bony tissue and an adequate compressibility of the vein, with good sensory feedback when puncturing with the needle. For veins manufacturing, a dipping technique with 2 layers of latex was selected, while for arteries, direct 3DP was preferred.
Soft tissues
Preliminary tests were also performed to evaluate the efficacy of Agar-based mixture as US transmission medium and in simulating patient’s soft tissues—that is, skin, muscles, and subcutaneous fat, according to the available literature.9,10 Unfortunately, the material exhibited an undesired behavior during puncture: the needle makes an agar coring that caps it and prevent the reflux of liquid inside its lumen; the liquid ascent—water in our case, blood in reality—represents the necessary confirmation of the simulated procedure’s success and the correct needle positioning inside the vessel. Moreover, Agar material gets easily damaged from repeated puncture, becoming unuseful after few uses. Lastly—being an organic material—it perishes in a short time: after few hours, the volume significantly decreases and within 48–72 h at room temperature it molds.
To overcome all the above-mentioned issues, a mixture of bi-component silicone (Ecoflex™ 00-10—with Part A and Part B mixed 1A:1B by weight or volume—Smooth-On Inc.) and Slacker® (a tactile mutator for platinum silicones) was tested as an alternative soft tissues’ simulation. The silicone-based mixture can guarantee longer lasting of the model over time, thanks to the absence of any organic substance. Moreover, the addition of a slacker percentage provides the silicone with elasticity and self-sealing features. The silicone and the slacker were mixed in equal parts. Previously described preliminary tests were repeated using the silicone-based mixture, leading the following results:
very realistic US visibility, improved with respect to the Agar-based medium;
good compressibility of soft tissues and vessels;
good self-sealing properties;
absence of coring of the material;
increased springback effect on needle puncture.
Stage 3: Phantom manufacturing
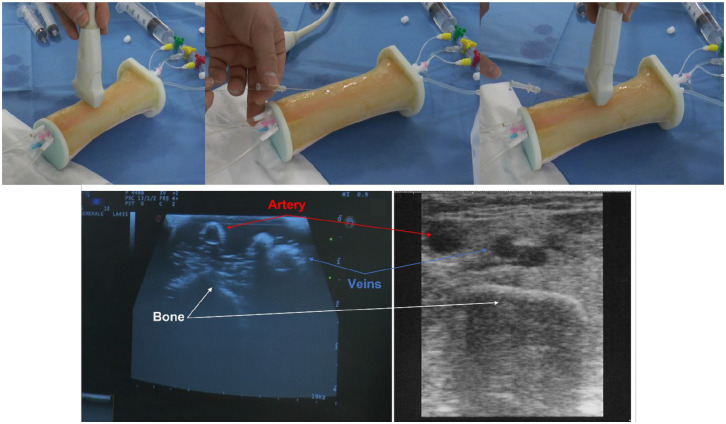
Once the most suitable materials and configurations for each structure of interest were selected, the patient-specific phantom was manufactured and assembled (Figure 3). To this aim, the skin was reconstructed from CT images and elaborated to create a 3D printed rigid mold. Vascular and bony structures were separately produced and placed into the mold, then poured with the silicone-based mixture. Vessels were designed to exceed the mold length, to fill them with water for US visibility.
Figure 3.
(Up) Final 3D printed pediatric phantom subjected to the real simulation of PICC insertion. (Down) Comparison between US images of 3D printed pediatric phantom (left) and real patient’s arm (right).
The first test on the patient-specific casted phantom highlighted a loss of echogenicity of vessels, due to a lack of crosslinking of the silicone around the latex vascular structures and a difficult sliding of the US probe on the silicone surface which need to be continuously hydrated with appropriate US-gel.
To overcome these issues: (i) the silicone mixture was poured on a 3D printed rigid vessels’ inner core (the same used for the latex dipping); only after silicone crosslinking, the cores were removed, and latex vessels inserted. Moreover, the mold design was modified to allow for horizontal placement, creating a wider surface exposed to air and promoting crosslinking; (ii) once hardened, the silicone mixture was coated with three latex layers simulating the skin: this allowed to significantly improve the US probe sliding on the phantom surface.
Stage 4: Phantom validation
A pilot study was conducted for the 3D printed patient-specific phantom validation. A total of 20 clinicians, experienced in PICC positioning procedure both on pediatric and adult patients, were asked to test the prototype, evaluate its morphology and the overall simulation experience. Clinicians performed (i) an US visual inspection to individuate the vascular structures, in particular basilica vein, and (ii) the US-guided puncture with the modified Seldinger technique: they had to insert both the needle and the guidewire for PICC positioning into the vein and then check the right positioning inside the vessel lumen through the US and the liquid reflux into the needle. To validate the model, they were asked to answer a specific 10-items questionnaire (Figure 4); results were then converted to a 5-point Likert scale. 11
Figure 4.
Evaluation questionnaire.
Results
Phantom manufacturing
Materials and manufacturing techniques employed for each 3D printed structure are summarized in Table 1.
Table 1.
Selected 3DP materials and technologies.
| Anatomical structure | Technique | 3D printer | Technology | Material |
|---|---|---|---|---|
| Homerus | Direct 3DP | ProJet 460 Plus | Binder Jetting | Chalk Powder |
| Arteries | Direct 3DP | ObJet260 Connex3 | Material Jetting | Photopolymer Resins |
| Veins | Dipping | — | — | Latex |
| Skin/mold | Direct 3DP | ObJet260 Connex3 | Material Jetting | Photopolymer Resins |
| Soft tissues | Molding | — | — | Silicone |
Model evaluation
A descriptive statistic was applied for demographic variables. The questionnaire’s variables were normally distributed in the Shapiro-Wilk test and were therefore reported as mean ± standard deviation. Validation data are summarized in Table 2. The model was rated highly realistic in terms of presentation, dimensions, mechanical behavior of vessels and soft tissues under puncturing, and overall simulation perception. US appearance of involved structures showed the lower score.
Table 2.
Evaluation questionnaires’ results.
| Mean | Std. Dev. | Std. Dev. % | |
|---|---|---|---|
| Presentation | 4.1 | 0.32 | 8 |
| Orientation | 4.4 | 0.44 | 10 |
| Anatomy | 3.7 | 0.41 | 11 |
| US appearance | 3.5 | 0.41 | 12 |
| Compressibility | 3.9 | 0.40 | 10 |
| Puncture | 4.2 | 0.47 | 11 |
| Strength | 4.3 | 0.38 | 9 |
| Fluid reflux | 4.3 | 0.47 | 11 |
| Seldinger | 4.4 | 0.37 | 8 |
| Overall evaluation | 4.2 | 0.34 | 8 |
Discussion
To date, the proposed 3D printed patient-specific phantom represents a pioneering perspective in pediatric surgical simulation and training. To the best of our knowledge, in the literature there are still no simulation models with such realistic features allowing to practice and improve gesture and manual skills in surgical maneuvers performed on pediatric patients.
One of the main issues to be faced was to find suitable materials that—on one hand—faithfully mimicked the US visibility of the arm’s anatomical structures and—on the other hand—had mechanical characteristics able to realistically reproduce vessels’ puncture and cannulation. A compromise between the realism of the US anatomical visibility and the puncture maneuver was needed. To improve the US visibility, the Agar-based mixture was replaced with additive silicone mimicking soft tissues. Various benefits came from this choice:
Agar is easily damaged by pricking, while the silicone mixture showed less deterioration, allowing to practice a higher number of procedures on the same phantom;
Silicone-Slacker mixture showed self-sealing properties;
While Agar is easily perishable, the mixture of silicone and Slacker is more durable. Moreover, an improved US visibility by hydrating the model with US-gel before and during the procedure was found;
Once the vessel is stung, the feedback given by the capillary reflux into the needle was achieved.
To achieve the complete silicone crosslinking, the latex vascular structures had to be inserted inside the phantom after the material’s pouring and complete solidification. In addition, it had to be poured horizontally for a wider surface area air exposition. To make the assembly possible, it was also necessary to eliminate the confluence between the brachial vein and the basilica vein. Moreover, the filling of only the upper half of the phantom led to the cephalic vein removal. This choice enabled a saving of more than 30% of the silicone, thus reducing costs.
According to the experts involved in the project, the phantom was rated good in terms of overall presentation (4.2/5), and able to faithfully reproduce the real physiological conditions.
As it concerns the tactile perception during venipuncture, very positive feedbacks were collected: the realism of the soft tissues and vessels’ consistency was greatly appreciated since operators achieved proper tactile feedback when the needle perforated the vessels. The liquid reflux by capillarity was considered realistic and could be further improved using a red dye. The Seldinger positioning was positively evaluated by all specialists too (4.4/5).
Main reported limitations were: (i) shadow-cones created by vessels on the US-image, making their identification too easy if compared to the reality; (ii) the horizontal silicone pouring created a hypoechoic surface area in the back, probably because of the absence of air trapped inside; (iii) the different compressibility of the brachial artery—produced through direct 3DP—with respect to the veins—produced through latex dipping technique—did not seem enough evident to discriminate between the different type of vessels. Possible solutions in this sense could be to increase the artery’s wall thickness and/or to use an infusion pump to simulate a pulsatile flow, therefore recognizable.
The overall production time—without considering the virtual model reconstruction and elaboration phases—was approximately 48 h.
The overall production cost was about €280.00, an easily affordable expense for any Institution aiming to organize training courses for their surgical teams. It is noteworthy to point out that the first printed phantom is still functional after more than 200 punctures, representing a very cost-saving device. Moreover, once the inner structures (i.e. artery and veins) will be too damaged for a high-fidelity simulation of the procedure—they will be replaced, but the 3D printed mold, together with the bony structure, the positioning bases and the skin support will be reused for a new silicone casting. This will lead to a significant decrease in production costs, from approximately €280.00 to about €80.00 (considering both the 3DP materials and poured silicone). On the contrary, there will not be great reduction in production times, as it is necessary to consider both the timing for the phantom’s assembly and the silicone cross-linking phases (more than 24 h). It is also important to underline that succeeding the procedure at the first attempt on the patient would avoid possible complications and the related additional costs.
Conclusions
Starting from real pediatric CT scans, a low-cost and high-fidelity 3D printed training arm model was created. To the best of our knowledge, this is the first example described in the literature. The proposed simulation phantom allows practicing the US-guided PICC positioning in children with high repeatability, safely improving individual performance in the procedure. The proposed simulator represents an important instrument for the organization of courses for students, trainees, or more experienced operators to learn or improve the execution of this maneuver.
This phantom could also serve as a base for the development of other 3D printed simulators for pediatric surgical training in our Institution, involving different pathological conditions and surgical interventions, leading to a new model of simulation-based medical education.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Italian Ministry of Health, RC-2019-945-rcr2019i2-17 “Patient-specific 3D-printing-based Simulation Platform for Live-Donor Robotic Nephrectomy and Transplantation.”
Ethical approval: A devoted ethical approval was not required for this study. The study does not involve the use of any biological sample or any change in the standard diagnostic and therapeutic path for patients. The use of sensitive data (computed tomography images) took place only within the same institution where they were acquired and stored. The images selected for the present study were anonymized and handled only by authorized personnel, according to the internal guidelines.
Informed consent: Informed consent acquisition complies with the Institution internal guidelines.
ORCID iDs: Alessandro Raffaele  https://orcid.org/0000-0002-5146-1486
https://orcid.org/0000-0002-5146-1486
Valeria Mauri  https://orcid.org/0000-0001-8458-6974
https://orcid.org/0000-0001-8458-6974
References
- 1. Mauro P, Scoppettuolo G. Manuale GAVeCeLT dei PICC e dei MIDLINE. 1st ed. Milan: Edra, 2016. [Google Scholar]
- 2. Emoli A, Cappuccio S, Marche B, et al. Il protocollo “ISP” (inserzione sicura dei PICC): un “bundle” di otto raccomandazioni per minimizzare le complicanze legate all’impianto dei cateteri centrali ad inserimento periferico (PICC). Assist Inferm e Ric 2014; 33(2): 82–89. [DOI] [PubMed] [Google Scholar]
- 3. Canzi P, Marconi S, Manfrin M, et al. From CT scanning to 3D printing technology: a new method for the preoperative planning of a transcutaneous bone-conduction hearing device. Acta Otorhinolaryngol Italica 2018; 38(3): 251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pietrabissa A, Marconi S, Negrello E, et al. An overview on 3D printing for abdominal surgery. Surg Endosc 2020; 34(1): 1–13. [DOI] [PubMed] [Google Scholar]
- 5. Auricchio F, Marconi S. 3D printing: clinical applications in orthopaedics and traumatology. EFORT Open Rev 2016; 1(5): 121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marone EM, Auricchio F, Marconi S, et al. Effectiveness of 3D printed models in the treatment of complex aortic diseases. J Cardiovasc Surg 2018; 59(5): 699–706. [DOI] [PubMed] [Google Scholar]
- 7. Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 2006; 31(3): 1116–1128. [DOI] [PubMed] [Google Scholar]
- 8. Sgromo V, Marconi S, Mauri V, et al. Echogenicity of 3D printing materials: a preliminary study for a new phantom-based ultrasound training. In “3D Printing and Biomechanics” 2nd CONGRESSO NAZIONALE IDBN & III thematic conference ESB-ITA, Pavia, 5–7 September 2018. [Google Scholar]
- 9. Earle M, Portu GD, DeVos E. Agar ultrasound phantoms for low-cost training without refrigeration. Afr J Emerg Med 2016; 6(1): 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Di Domenico S, Licausi M, Porcile E, et al. Introduzione nella pratica clinica del cateterismo venoso centrale ecoguidato: una guida per realizzare un percorso di apprendimento con economici modelli artigianali. J Ultrasound 2008; 11: 135–142.23396222 [Google Scholar]
- 11. Nemoto T, Beglar D. Developing Likert-scale questionnaires. In: JALT2013 conference proceedings (eds Sonda N, Krause A.), Virtual, 2014, pp.1–8. Tokyo: JALT. [Google Scholar]