Abstract
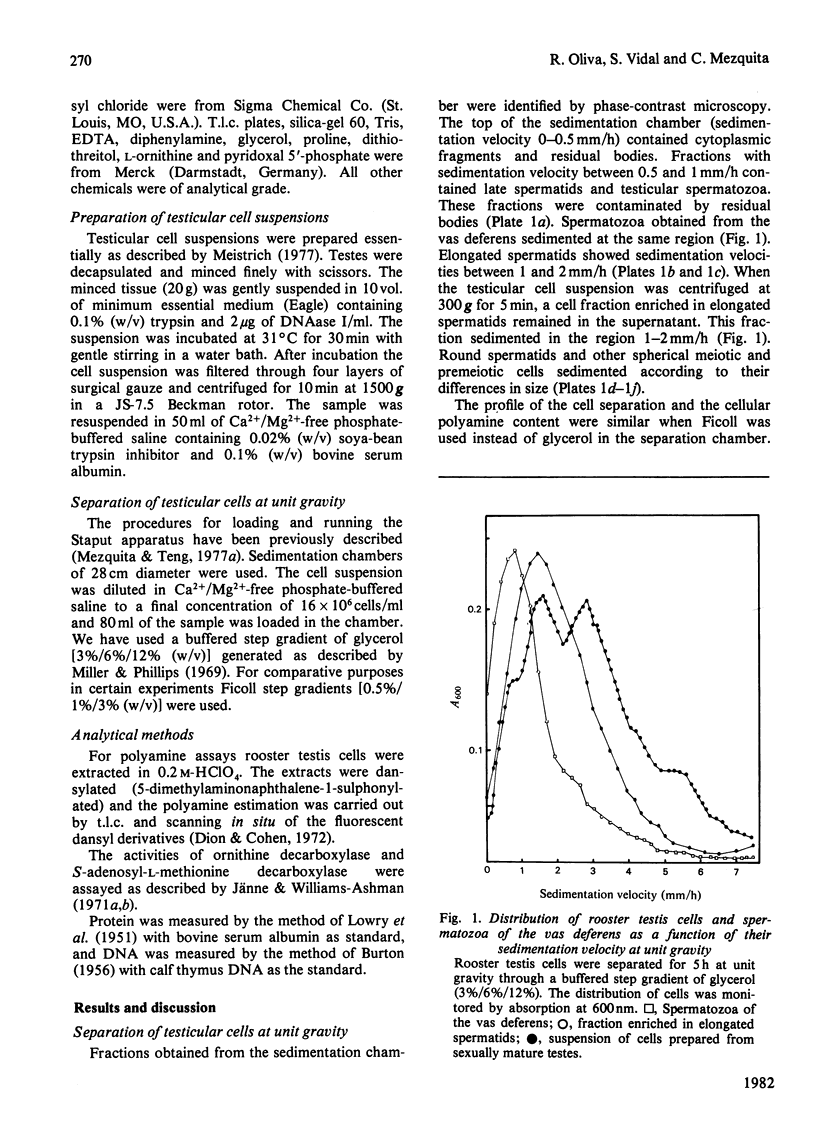
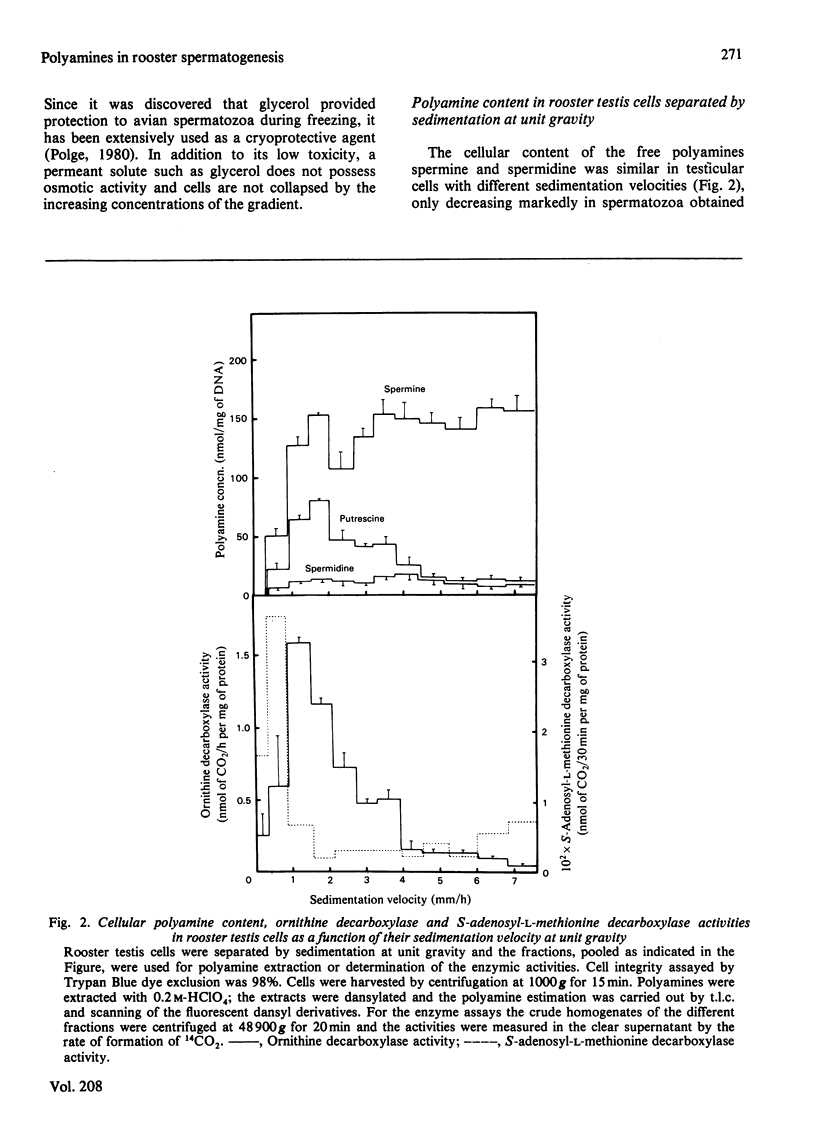
The natural polyamines spermine and spermidine, and the diamine putrescine, were extracted from rooster testis cells separated by sedimentation at unit gravity, and from vas-deferens spermatozoa. The ratios spermine/DNA and spermidine/DNA were kept relatively constant throughout spermatogenesis, whereas the ratio putrescine/DNA rose in elongated spermatids. The cellular content of spermine, spermidine and putrescine decreased markedly in mature spermatozoa. Two rate-limiting enzymes in the biosynthetic pathway of polyamines, ornithine decarboxylase and S-adenosyl-L-methionine decarboxylase, showed their highest activities at the end of spermiogenesis and were not detectable in vas-deferens spermatozoa. A marked reduction in cell volume during spermiogenesis without a parallel decrease in the cellular content of polyamines suggests the possibility that the marked changes in chromatin composition and structure occurring in rooster late spermatids could take place in an ambience of high polyamine concentration.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alhonen-Hongisto L. Regulation of S-adenosylmethionine decarboxylase by polyamines in Ehrlich ascites-carcinoma cells grown in culture. Biochem J. 1980 Sep 15;190(3):747–754. doi: 10.1042/bj1900747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellona W. J., Brackeen R. B., Brinkley B. R. Differential binding of tritiated actinomycin to the nuclei of mammalian spermatogenic cells in vivo. J Reprod Fertil. 1974 Jul;39(1):41–48. doi: 10.1530/jrf.0.0390041. [DOI] [PubMed] [Google Scholar]
- Clarke C. H., Shankel D. M. Antimutagenesis in microbial systems. Bacteriol Rev. 1975 Mar;39(1):33–53. doi: 10.1128/br.39.1.33-53.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion A. S., Cohen S. S. Polyamines in the synthesis of bacteriophage deoxyribonucleic acid. I. Lack of dependence of polyamine synthesis on bacteriophage deoxyribonucleic acid synthesis. J Virol. 1972 Mar;9(3):419–422. doi: 10.1128/jvi.9.3.419-422.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosule L. C., Schellman J. A. Compact form of DNA induced by spermidine. Nature. 1976 Jan 29;259(5541):333–335. doi: 10.1038/259333a0. [DOI] [PubMed] [Google Scholar]
- JAENNE J., RAINA A., SIIMES M. SPERMIDINE AND SPERMINE IN RAT TISSUES AT DIFFERENT AGES. Acta Physiol Scand. 1964 Dec;62:352–358. doi: 10.1111/j.1748-1716.1964.tb10433.x. [DOI] [PubMed] [Google Scholar]
- Jänne J., Pösö H., Raina A. Polyamines in rapid growth and cancer. Biochim Biophys Acta. 1978 Apr 6;473(3-4):241–293. doi: 10.1016/0304-419x(78)90015-x. [DOI] [PubMed] [Google Scholar]
- Jänne J., Williams-Ashman H. G. Dissociation of putrescine-activated decarboxylation of S-adenosyl-L-methionine from the enzymic synthesis of spermidine and spermine by purified prostatic enzyme preparations. Biochem Biophys Res Commun. 1971 Jan 22;42(2):222–229. [PubMed] [Google Scholar]
- Jänne J., Williams-Ashman H. G. On the purification of L-ornithine decarboxylase from rat prostate and effects of thiol compounds on the enzyme. J Biol Chem. 1971 Mar 25;246(6):1725–1732. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loir M., Hochereau-de Reviers M. T. Deoxyribonucleoprotein changes in ram and bull spermatids. J Reprod Fertil. 1972 Oct;31(1):127–130. doi: 10.1530/jrf.0.0310127. [DOI] [PubMed] [Google Scholar]
- Loir M., Lanneau M. Transformation of ram spermatid chromatin. Exp Cell Res. 1978 Sep;115(2):231–243. doi: 10.1016/0014-4827(78)90277-x. [DOI] [PubMed] [Google Scholar]
- MacIndoe J. H., Turkington R. W. Hormonal regulation of spermidine formation during spermatogenesis in the rat. Endocrinology. 1973 Feb;92(2):595–605. doi: 10.1210/endo-92-2-595. [DOI] [PubMed] [Google Scholar]
- Meistrich M. L. Separation of spermatogenic cells and nuclei from rodent testes. Methods Cell Biol. 1977;15:15–54. doi: 10.1016/s0091-679x(08)60207-1. [DOI] [PubMed] [Google Scholar]
- Mezquita C., Teng C. S. Studies on sex-organ development. Changes in chromatin structure during spermatogenesis in maturing rooster testis as demonstrated by the initiation pattern of ribonucleic acid synthesis in vitro. Biochem J. 1978 Feb 15;170(2):203–210. doi: 10.1042/bj1700203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezquita C., Teng C. S. Studies on sex-organ development. Changes in nuclear and chromatin composition and genomic activity during spermatogenesis in the maturing rooster testis. Biochem J. 1977 Apr 15;164(1):99–111. doi: 10.1042/bj1640099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. G., Phillips R. A. Separation of cells by velocity sedimentation. J Cell Physiol. 1969 Jun;73(3):191–201. doi: 10.1002/jcp.1040730305. [DOI] [PubMed] [Google Scholar]
- Rajalakshmi S., Rao P. M., Sarma D. S. Studies on carcinogen chromatin--DNA interaction: inhibition of N-methyl-N-nitrosourea-induced methylation of chromatin--DNA by spermine and distamycin A. Biochemistry. 1978 Oct 17;17(21):4515–4518. doi: 10.1021/bi00614a024. [DOI] [PubMed] [Google Scholar]
- Rosenheim O. The Isolation of Spermine Phosphate from Semen and Testis. Biochem J. 1924;18(6):1253–1262.1. doi: 10.1042/bj0181253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]



