Abstract
Background
The efficacy of lung cancer screening (LCS) to reduce lung cancer specific mortality is heavily dependent on adherence to recommended screening guidelines, with real-world adherence rates reported to be drastically lower than rates described in clinical trials. There is a dearth in the literature on reminder processes and clinical workflows used to address adherence and robust data is needed to fully understand which clinical set-ups, processes, and context enhance and increase continued LCS participation. This paper describes a protocol for an environmental scan of adherence and reminder processes that are currently used in LCS programs across the United States.
Methods
This study will triangulate data using a 3-step explanatory sequential mixed methods design to describe mechanisms of current adherence and reminder systems within academic and community LCS programs to pinpoint clinic or system barrier and facilitator combinations that contribute to increased adherence. In step 1, surveys from a nationally representative sample of LCS programs will yield quantitative data about program structure, volume, and tracking/reminder processes and messages. After completion of the survey, interested LCS program personnel will be invited to participate in an in-depth interview (step 2) to explore current processes and interventions used for adherence at the participant and program level. Finally, in step 3, triangulation of quantitative and qualitative data will be completed through qualitative comparative analysis to identify combinations of components that affect higher or lower adherence.
Discussion
This research advances the state of the science by filling a gap in knowledge about LCS program characteristics and processes associated with better adherence which can inform the development and implementation of interventions that are scalable and sustainable across a wide variety of clinical practice settings.
Keywords: Lung cancer screening, Contextual factors, Qualitative comparative analysis, Mixed-methods, Adherence
Contributions to the literature .
Adherence to annual lung cancer screening guidelines is critically low, hindering the individual and population health benefits associated with early detection of lung cancer.
There is currently poor understanding of contextual factors at the clinic and healthcare system levels that influence adherence rates in academic, community, and rural lung cancer screening programs.
This mixed-methods, environmental scan study will provide needed knowledge on adherence processes in diverse practice settings, and second, inform future adherence and reminder intervention development and implementation research.
Background
Context is undoubtedly an integral component of implementation science, affecting the success of both initial implementation and continued sustainment of an intervention [1, 2]. While there are varying definitions of context available in the literature, it is widely accepted that context consists of much more than the implementation setting [1–5]. Context can be influenced by a variety of factors, including health care policy, payment and reimbursement systems, practice and health system organization and priorities, and characteristics of the target population [3], and has been described by Øvretveit, et al. to include “everything else that is not the intervention” [6] (p.605). Given the complex and dynamic nature of context, factors that act as facilitators in one setting may act as a barrier in another setting [2].
Measuring and understanding context has emerged as a priority for the maturing and evolving field of implementation science, with many widely-used implementation science frameworks addressing contextual dimensions [4, 5]. The Agency for Healthcare Research and Quality recommends that attention be paid to context during all stages of research, including planning, implementation, analysis, and reporting [3]. Furthermore, dimensions of context are often interdependent, and successful implementation may depend on combinations of contextual factors [4]. A multilevel, participatory approach working directly with relevant stakeholders is best suited to identify, monitor, and report contextual factors that influence meaningful outcomes and equitable scalability [3].
Assessing context for lung cancer screening adherence
Lung cancer screening (LCS) with low-dose computed tomography (LDCT) holds tremendous potential to transform lung cancer outcomes. When diagnosed late, lung cancer has a five-year survival rate of 9%, but exceeds 60% when diagnosed early [7]. Only 22% of lung cancers are currently diagnosed early [7], but have increased by 4.5% per year since LCS received a grade B recommendation by the US Preventive Services Task Force (USPSTF) in late 2013 [8, 9]. When diagnosed at an early stage, lung cancer is potentially curable with more effective treatment options available leading to reduced mortality and improved quality of life [10, 11]. Two large clinical trials have provided evidence that LCS reduces lung cancer mortality by at least 20% when performed annually,10,11. Despite this clinical trial evidence and federal policy support, LCS remains vastly underutilized among eligible individuals [12]. USPSTF currently recommends LCS for individuals who are 50–80 years of age, have a tobacco exposure history of 20 pack years or more, and currently smoke cigarettes or have quit within the past 15 years [13].
Adherence to recommended follow-up is vitally essential to maximize the efficacy of LCS [14], with approximately 90% of screened individuals having recommended annual screening follow-up (Lung-RADs 1 or 2 results) [15]. The remaining 10% will have a result that is worrisome for lung cancer and require shorter-term follow-up (Lung-RADs 3 or 4) [15]. The lung cancer-specific mortality reduction reported in clinical trials can be partially attributed to the adherence rates that exceed 90% over multiple rounds of screening [10, 16], a necessary attribute of cancer screening that has not transferred to non-clinical trial settings [17, 18]. Real-world annual adherence rates have been routinely reported to be less than 50% [17, 18] and as low as 22% [19]. Reports of adherence to shorter-term follow-up are also low (< 60%) [20]. More importantly, adherence rates for LCS are lower for racial and ethnic minority populations [20–22], revealing a critical gap in achieving optimal population-based and health equity-focused LCS efficacy.
Prior LCS adherence research has been predominantly quantitative and single-center in nature [23–28], hindering the generalizability and reproducibility to more diverse practice settings. One variable consistently associated with better adherence is a centralized program structure that uses a navigator or coordinator to oversee the entire LCS process for providers and participants [23, 24]. Additionally, reminder messages have been vital for increasing adherence rates in cervical, breast, and colorectal cancer screenings [29, 30], and have been reported to be used in many LCS programs as well [26, 29, 31]. There is a dearth in the literature on reminder processes and clinical workflows used to address adherence. In this capacity, robust qualitative data is needed to fully understand which clinical set-ups, processes, and context enhance and increase continued LCS adherence.
Reminder messages provide an important opportunity to engage with LCS participants and communicate the significance of continued participation. Reading levels for LCS educational resources have primarily consisted of levels above those recommended by the American Medical Association and the National Institutes of Health (below 6th or 7th grade) [32, 33]. The content and reading level of reminder message templates used in clinical LCS programs is currently unknown. Understanding these nuances will influence how adherence and reminder interventions can optimally be designed, implemented, and tested for sustainable and scalable dissemination while addressing equity for all.
Environmental scanning
This paper describes a protocol for an environmental scan of adherence and reminder processes that are currently used in LCS programs across the United States. Environmental scanning originated in the business sector to analyze internal strengths and challenges with external influences to improve organizational strategies and performance [34, 35]. Environmental scanning has been used in the public health, health services delivery, and medicine fields to examine the state of programs, practices, or services and to identify organizational/community needs, barriers, service, and research gaps [34–37]. The purposes of this environmental scan are to first provide needed knowledge on adherence processes in academic and community LCS programs and second, to inform future adherence and reminder intervention development and implementation research.
Successful intervention implementation and continued sustainment require that the intervention fits the context of the targeted clinical environment [38]. Pragmatic study design and implementation science strive to close the gap between clinical research and practice by engaging health care systems, clinicians, and patients in the research process in order to understand the context of the clinical setting and how to successfully implement scalable interventions that meet the needs and desired outcomes of the stakeholders [39]. Clinical and health system context has predominantly been measured with quantitative or qualitative methods alone [5]. Mixed methods approaches integrating quantitative and qualitative data lead to a better understanding of the question and evidence [40]. This environmental scan will employ an implementation science, mixed methods design to describe mechanisms of current adherence and reminder systems within academic and community LCS programs to pinpoint clinic or system barrier and facilitator combinations that contribute to increased adherence.
Methods
Design overview
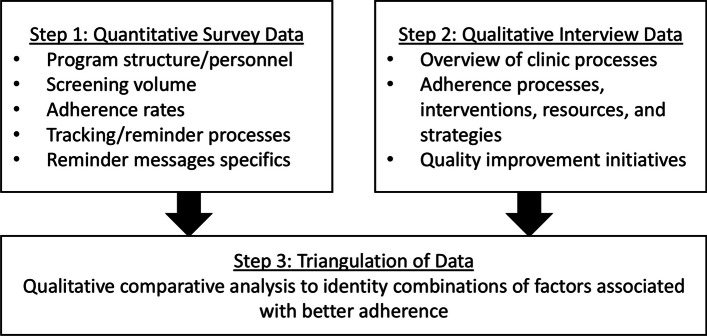
This study will triangulate data using a 3-step explanatory sequential mixed methods design [40] to provide insight into adherence, tracking, and reminder context and interventions, as summarized in Fig. 1. In step 1, a cross-sectional survey from a nationally representative sample of LCS programs will yield quantitative data about program structure, volume, and tracking/reminder processes and messages. After completion of the survey, interested LCS program personnel will be invited to participate in an in-depth interview (step 2) to qualitatively explore current processes and interventions used for adherence at the participant and program levels. Finally, in step 3, triangulation of quantitative and qualitative data will be completed through qualitative comparative analysis to identify combinations of components that affect higher or lower adherence. The reporting of Steps 1 and 2 conform to CROSS guidelines [41] and COREQ guidelines [42], respectively.
Fig. 1.
Three-step data collection and design overview
Theoretical and methodologic underpinnings
This research was conceptualized and guided by Choo’s conceptual model of environmental scanning [34]. Choo's model posits that environmental scanning can be used to look at information (viewing) or look for information (searching), and four types of scanning exist (undirected viewing, enacting, conditioned viewing, and searching), dependent on the availability of information to understand the complexity of the environment and to what extent the organization or researcher intervenes to understand the environment [34]. A scoping review that applied Choo's model to environmental scanning in the health services delivery field found that two of the four scanning types, conditioned viewing and searching, were relevant to health services delivery research [35]. Conditioned viewing is applied by using passive data collection to look at information, and searching uses active data collection to look for information [35]. This study will use the searching scanning type described by Choo to actively engage with LCS personnel and actively collect data with the use of surveys and interviews.
An implementation science approach will be leveraged by partnering directly with LCS program personnel to describe real-world clinical context and processes, providing unparalleled insight into multilevel challenges and priorities of screening programs across the country [43]. This study will also leverage strategies commonly used to strengthen implementation research, including using a mixed methods analysis and addressing multiple levels of the health care delivery system, mainly the clinic/provider and system/organizational levels. A multilevel perspective is important to best understand the interdependence and influence of each level on LCS adherence [44].
Mixed methods data integration of quantitative survey data and qualitative interview data will be completed using qualitative comparative analysis (QCA). QCA is a set-theoretic research methodology for analyzing causal complexity, situations in which combinations of conditions may combine in various ways to produce or prevent an outcome [45]. Best suited for use in small or medium-sized (n = 10 – 50) case studies, QCA can combine case-oriented (qualitative) and variable-oriented (quantitative) data to understand real-world clinical circumstances [46]. Originally developed in the comparative politics and macrosociology fields, QCA use has emerged in the public health field as a strategy to evaluate complex relationships of intervention components and dynamic environmental context [47]. For this research, the team is interested in determining which combinations of LCS program characteristics and adherence processes lead to better annual adherence.
Step 1 quantitative survey
Participants
Participants for step 1 will include LCS program navigators associated with the GO2 for Lung Cancer Screening Centers of Excellence (SCOE), a population of screening programs dedicated to providing the highest quality LCS, supporting the translation of evidence-based guidelines into actual clinical practice [48]. GO2 SCOE represents community, academic, and VA programs in 45 states and Washington, DC. The mission and breadth of programs make GO2 an unparalleled partner for supporting LCS implementation research studies. All SCOE navigators/coordinators who are at least 18 years of age, can complete surveys and interviews in English and are willing to document informed consent will be eligible to participate. Navigators/coordinators who are unwilling to document informed consent will be excluded.
Procedures
Recruitment
Step 1 survey participants will be recruited through the GO2 for Lung Cancer SCOE. A study introduction and electronic survey link will be sent to email contact information by the GO2 research team for all SCOE coordinator contacts. Personnel from all programs (~ 900 SCOE) will be invited to participate in the survey if they meet the inclusion criteria. Recipients will be asked to forward the survey to the appropriate person if they are not the primary contact for their LCS program.
Informed consent and retention
An informational consent sheet available in REDCap (Research Electronic Data Capture; Vanderbilt University; electronic data capture hosted at Hackensack Meridian Health) will include information about the risks and benefits of the study and contact information for the research team will be available for any questions from potential participants. Interested participants will need to read and acknowledge the contents of an informational consent form available in REDCap to formally participate in the study.
Survey distribution and data collection
Survey respondents will immediately have the opportunity to complete the survey after agreeing to the contents of the informational consent sheet. At survey completion, demographic and characteristic data will be collected from each participant, including sex, ethnicity, race, years of LCS experience, and education. Participants will have the option to provide their name and email at this point to be entered into the gift card drawing if desired. Survey respondents will also be asked to voluntarily upload or email reminder message templates used by their program for use in a content and readability analysis. Sharing reminder templates will be optional, and survey respondents will be encouraged to remove any identifying program information if it would make them more comfortable. No identifying program information will be recorded or used as part of the content and readability analyses.
Principles of the Tailored Design Method [49] will be used to maximize the response rate and encourage participation. Prospective participants will be contacted up to three times over a four-week period, and respondents will have the option to enter into a drawing to receive one of ten $50 Amazon gift cards upon survey completion.
Any risks to the participants enrolled in all aims of this study will be minimal. No stigmatizing or confidential data will be collected during the surveys or interviews. The amount of identifiable information collected will be minimal (name and contact) for scheduling purposes only and any identifiers will be destroyed as soon as possible. All survey data will be stored in a secure REDCap database and survey respondents will be assigned a unique study identification number that is only available to study investigators.
Measures
The step 1 survey was informed through environmental scan and capacity instruments that have been used for previous cancer screening [50–54] research with additional items specific for LCS prioritized through the expertise of the research team. The survey is divided into eight sections with questions addressing 1) program history and structure, 2) workflows and processes, 3) no-show tracking and reminders, 4) annual adherence (Lung-RADs 1 or 2 results) tracking and reminders, 5) Lung-RADs 3 (small nodule that is monitored with a 6-month LDCT) tracking and reminders, 6) Lung-RADs 4 (nodule that is more worrisome for lung cancer required shorter-term follow-up) tracking and reminders, 7) details about current reminder messages, and 8) respondent demographics and characteristics (Appendix). Questions related to program history, structure, workflows, and processes were adapted from survey work that has been completed by the Project ACTS (Adherence to CT Screening) [55] research team (personal communication with Drs. JL Studts, MM Byrne, and U Basu Roy). The survey instrument was pre-tested for length of time for completion and question clarity by all study authors and three research coordinators and associates with LCS expertise.
The reminder message template content and readability analyses will be guided by similar work performed on LCS educational websites [32, 33, 56] and key LCS communication recommendations [57]. An initial content analysis data extraction sheet will be iteratively refined as needed to collect information on organizational and logistical support, social support, educational aspects, LCS benefits, LCS risks, and costs [32, 57]. All reminder templates will be coded using the final version of the data extraction sheet. The readability of written information on each reminder template will be assessed using 3 validated readability assessment tools: the Flesch-Kincaid grade level [58], the Simple Measure of Gobbledygook index [59], and the Gunning-Fog Score [60]. The Flesch-Kincaid score is based on the average number of words per sentence and the mean number of syllables per word [58]. The Simple Measure of Gobbledygook selects 10 sentences from the beginning, middle, and end of a text and uses the number of words with 3 syllables or more [59]. The Gunning-Fog index tabulates reading level with a formula using the number of sentences, the number of words, and the number of words with 3 syllables or more [60].
Sample size
The research team aims to have a minimum of 315 completed surveys (~ 35% response rate) since navigators/coordinators from all SCOE programs will be invited to complete the survey. Utilizing a two-sample proportion test and assuming a minimum sample size of 315 survey respondents, we will have 80% power to detect a minimum difference of 16% for a χ2 test with one degree of freedom or 17.5% for a χ2 test with two degrees of freedom, between program characteristics and reminder processes at an alpha level of 0.05. Power calculations were performed using PASS 11 (NCSS, LLC) [61].
Analysis
Data will be analyzed with SAS/STAT, v9.4 (SAS Institute Inc., Cary, NC, USA) and will use duplicate data checking macros available in SAS to check for duplicate records [62]. Survey data will be summarized with descriptive statistics, depending on the distribution of the collected data. Univariate differences between program characteristics and adherence processes will be tested with a t-test or ANOVA for continuous variables (or non-parametric tests) and χ2 test for categorical variables. Missing data will also be assessed, including rate of missing data and type or missing data. It is expected that any missing data will be missing at random and that pairwise deletion will be used to remove records with missing values for analyses of that variable only [63].
Descriptive statistics will be used to characterize the informational content and readability of each LCS reminder template. The medians and standard deviations of reminder template readability scores, word count, and reading time will be calculated, and scatterplots and/or boxplots will be generated to show variability in these scores and word counts.
Step 2 qualitative key informant interviews
Participants
A subset of 50 participants (~ 25 each from LCS programs with higher and lower annual adherence) will be invited to participate in a key informant interview to garner additional insight into the workflows and methods programs are using to address adherence. As there is not an existing definition for high or low LCS annual adherence, groups for higher and lower adherence will be divided based on rates reported by the LCS program in the step 1 survey.
Procedures
Recruitment
Step 2 interview participants will be identified from LCS navigators/coordinators who completed the Step 1 survey. The survey will contain a brief synopsis and question about continued involvement in the key informant interview for step 2. Inclusion and exclusion criteria will be consistent with step 1 survey criteria.
Informed consent and interview procedures
The research team will contact individuals interested in completing the interview with additional information and to determine eligibility. Similar to step 1, informed consent procedures will consist of agreeing to the contents of an electronic REDCap informational consent sheet. After informed consent acknowledgment, interviews will be conducted by an experienced, doctorally trained female interviewer (EAH) via Zoom. The interviewer will not be known to the participants prior to the interview and details about the LCS background and interest in adherence of the interviewer and research study will be shared at the beginning of the interview, prior to starting the formal interview procedures. The interview will be conducted using cognitive interviewing principles (comprehension, retrieval from memory or relevant information, decision/judgment properties, and response processes) [64]. Probes will be used when appropriate, and interviews will be recorded/transcribed. Since the interviews will be conducted using Zoom, the researchers will have little control over the interview setting and whether other individuals may be present. Interview participants will be informed that interviews are meant to be conducted 1-on-1, they can choose to have their cameras off during the interview if desired and will be compensated with a $20 Amazon gift card upon completion of the interview.
Interview guide and measures
The semi-structured interview guide (Appendix) is aligned with select constructs of the Consolidated Framework for Implementation Research (CFIR) [65] that have been reported to influence LCS implementation at the system and provider/clinic level [66]. CFIR is one of the most extensive implementation frameworks available and is widely used to evaluate implementation barriers and facilitators [67, 68]. Interview questions were adapted from relevant questions for each CFIR domain available in the CFIR Interview Guide Tool (https://cfirguide.org/guide/app/). (Appendix).
Sample size
Step 2 includes 50 interviews, 25 each from programs with higher and lower adherence. Interviews will be stopped early if saturation is reached or continue beyond 50 if saturation has not been reached.
Analysis
Qualitative data will be coded by thematic analysis [69] and summarized by themes surrounding the burden to workflow, barriers, and facilitators of adherence processes. Two coders will complete coding for at least the first five interviews to monitor intrareader reliability. Coding will be inductive, and standardized codes from the first five interviews will be used to inform the development of a codebook to subsequently code the remaining interviews. The study team will meet several times during the coding process to review codes and determine when saturation is reached. Codes and themes will further be used in qualitative comparative analysis.
Step 3 Qualitative Comparative Analysis (QCA)
The QCA analysis will require three steps. First, calibration of conditions (variables) is completed to determine set membership criteria for qualitative and quantitative elements [70]. Membership is defined as 0 ≤ x ≤ 1, where a score of 0 means the condition is clearly out of the target set, and a score of 1 means the condition is clearly in the target set; scores within this interval indicate fractional set membership. The second step is a necessity analysis that identifies whether any conditions are required for the outcome's occurrence [70]. Finally, a sufficiency analysis is conducted in order to identify and distinguish those combinations of conditions (“explanatory recipes”) that consistently are associated with the presence of the outcome from those that are not [70]. Data triangulation through qualitative comparative analysis will require complete data from 40 matched surveys and interviews.
Ethics approval and informed consent
This research was approved by the Hackensack Meridian Institutional Review Board (Pro2023-0267) with a waiver of written documentation of informed consent. Informational consent sheets will be available in REDCap and contain information about the study's purpose, procedures, risks, and benefits. Study participants will be encouraged to print the information sheet for their records. The recruitment and informed consent procedures will preserve participants' right to refuse and guarantee that the research team will not know potential participants who refuse.
Discussion
Results from this research will provide much-needed environmental scan data on LCS adherence from a nationally representative sampling of screening programs. Low annual adherence has been recognized as a critical challenge in reaching optimal population health benefits of LCS [14]. Published adherence rates have been reported to be better in some programs (> 60%) [27, 71, 72], and it may be possible that mechanisms used to address adherence in these programs can be implemented into LCS programs with lower adherence. This research helps fill a gap in knowledge about LCS program characteristics and processes associated with better adherence, helping lead to the development and implementation of scalable and sustainable interventions across a wide variety of clinical practice settings.
Successful sustainment of new interventions depends on the ability of the intervention to meet the priorities of the clinical team, to become part of routine clinical practice, and for ongoing costs to fit into operating budgets [73]. This study uses innovative methods to advance current knowledge and understanding of real-world LCS clinical practice and context. A comprehensive mixed-methods approach will collect essential key factors related to LCS adherence across the nation in academic, community, and VA programs. Data triangulation and integration of quantitative survey data and qualitative interview themes through the application of QCA will add to the literature in new and insightful ways by addressing what combinations of factors lead to improved adherence in a complex clinical environment [45].
Additionally, this study will perform a content and readability analysis on reminder message templates currently used by LCS programs. Health reminders are an avenue to enhance relationships with healthcare systems and provide educational opportunities [74]. Sending reminder messages offers an additional opportunity for LCS programs to educate screening participants about the importance of continued LCS participation [75]. Realizing that a substantial proportion of the adult population in the United States have lower health literacy, the Agency for Healthcare Research and Quality recommends that clinical practices perform readability and understandability audits on materials used to communicate with patients, including appointment reminders and educational fact sheets [76]. Since low health literacy is associated with both poorer health outcomes [77] and is more common among populations that are more likely to smoke cigarettes (i.e., rural residence, racial/ethnic minorities, low socioeconomic status) [78, 79], a better understanding of the content and reading level of reminder messages is needed to support reminder interventions and participant engagement opportunities.
While this environmental scan will be strengthened with an implementation science and mixed methods approach, some limitations must be recognized using the available recruitment strategies and methodologies. First, recruitment is being conducted with a single navigator community that requires multilevel organizational support and resources to provide high-quality LCS to be designated an SCOE [48]. Some of the required organizational supports, such as access to a lung cancer multidisciplinary team and internal quality improvement process, may not exist in lower resource settings and results from this research may not apply to all LCS clinical settings. Second, there are multiple aspects of the reminder process that the survey is detailed to capture, including mode, timing, frequency, who sends the reminder, and to whom the reminder is sent. While this detailed information will provide a comprehensive snapshot of reminder processes, the granularity of the survey may be burdensome for busy LCS program personnel. The research team attempted to lessen the possible burden by shortening the survey length and pre-testing the survey with individuals with LCS expertise to obtain an accurate estimate of survey completion time. Despite these limitations, this research is among the first research studies grounded in implementation science to identify system and clinic influences of LCS adherence and reminder processes. The approach will provide valuable information on mechanisms of better adherence in settings outside of clinical trials and will shape future LCS adherence intervention development, implementation, and sustainment.
Acknowledgements
We thank Dr. Claude Rubinson from the University of Houston Downtown for his guidance on the use of qualitative comparative analysis in this research work.
Abbreviations
- CFIR
Consolidated Framework for Implementation Research
- LCS
Lung cancer screening
- LDCT
Low-dose computed tomography
- QCA
Qualitative comparative analysis
- REDCap
Research Electronic Data Capture
- SCOE
GO2 for Lung Cancer Screening Centers of Excellence
- USPSTF
United States Preventive Services Task Force
Appendix
Overview of questions for step 1 quantitative survey
| Program history/structure |
• Year of program initiation • Number of personnel and navigators/coordinators • Medical specialties involved with operations • Medical specialty of program Medical Director • Structure (centralized/hybrid/decentralized) • Service area (urban, suburban, rural) • Practice setting (Large hospital, small hospital, VA, academic, cancer center, outpatient radiology, other) • Zip code of screening program |
| Overall workflows and processes |
• Source of referrals (internal or external primary care, pulmonary, other medical specialty) • Performance of shared decision-making • Types of affiliated imaging centers (internal or external centers located in hospital or free-standing centers) • Availability of CT scans for after-hours or weekend appointments • Procedure wait time (Annual CTs, 6-month CTs, PET/CT, tissue sampling • Volume of low-dose CTs in past year • Tracking platform used (Excel, Word, electronic health record, commercially available software) ◦ If applicable, which tracking software is used • Capabilities of tracking software |
| No-shows tracking and reminders |
• Does program track no-shows to the low-dose CT appointment • No-show rate • No-show reminders sent to screening participants ◦ Number of reminders sent (0, 1, 2, 3+) ◦ Who sends reminders (for each reminder sent) ◦ Modality of reminder (for each reminder sent) ◦ Timing of reminder (for each reminder sent) • No-show reminders sent to primary care or ordering provider ◦ Number of reminders sent (0, 1, 2, 3+) ◦ Who sends reminders (for each reminder sent) ◦ Modality of reminder (for each reminder sent) • Timing of reminder (for each reminder sent) |
| Annual adherence, Lung-RADs 3, and Lung-RADs 4 tracking and reminders |
• Annual adherence rate • Shorter-term (interval) adherence rate • Annual/Lung-RADs 3/Lung-RADs 4 reminders sent to screening participants: ◦ Number of reminders sent (0, 1, 2, 3+) ◦ Who sends reminders (for each reminder sent) ◦ Modality of reminder (for each reminder sent) ◦ Timing of reminder (for each reminder sent) • Annual/Lung-RADs 3/Lung-RADs 4 reminders sent to primary care or ordering provider ◦ Number of reminders sent (0, 1, 2, 3+) ◦ Who sends reminders (for each reminder sent) ◦ Modality of reminder (for each reminder sent) ◦ Timing of reminder (for each reminder sent) |
| Details about reminder messages currently used |
• How reminder messages were developed • Capability of LCS program to send messages by text message, email, through electronic health record • Health equity, health literacy, or imaging aspects of messages |
| Respondent demographics and characteristics |
• Age • Gender • Employment status • Education • Race • Ethnicity • Percent work effort allocated to LCS program • Years of LCS experience • Length of time working with current LCS program |
Adapted CFIR interview questions (Step 2)
| System and Clinic Level Barriers and Facilitators Adapted to Address Adherence | CFIR Guide Root Interview Questions | Adapted Interview Question |
|---|---|---|
| Intervention Characteristics | ||
|
System Level Implications Barriers: Process and cost of tracking Facilitators: Efficacy of need for optimal adherence well documented from clinical trial evidence Provider/clinic Level Implications Barriers: Process and cost of tracking Facilitators: Efficacy of need for optimal adherence well documented from clinical trial evidence |
What do influential stakeholders think of the intervention? | In your opinion, what are the most important aspects of a successful lung cancer screening program? How do you define success for a lung cancer screening program? |
| Please consider the following aspects of the intervention: duration, scope, intricacy and number of steps involved and whether the intervention reflects a clear departure from previous practices | If you could wave a magic wand, is there anything you change about the work-flow of your LCS program overall? Specific to adherence? | |
| What costs will be incurred to implement the intervention? | Is cost a current barrier for your magic wand idea? Do you believe cost is a prohibitive parameter for success of your program? Implementation procedures? Adherence? | |
| Outer Setting | ||
|
System Level Implications: Barriers: Insurance and reimbursement issues Facilitators: None Provider/clinic Level Implications: Barriers: Logistical and cost barriers for LCS participants Facilitators: Barrier reduction and understanding of population being served by LCS program |
To what extent were the needs and preferences of the individuals served by your organization considered when deciding to implement the intervention? | To what extent were the needs and preferences of your LCS population considered when deciding on your adherence procedures? Reminder materials? How about the needs and preferences of your ordering providers? |
| Have you elicited information from participants regarding their experiences with the intervention? | Have you or your health system asked your LCS participants regarding their experiences with your tracking or reminder procedures? | |
| Have you heard stories about the experiences of participants with the intervention? | Are you familiar with barriers your LCS participants’ experience that may keep them from being adherent to annual screening/follow-up? Does your program have any programs or solutions in place to help address these barriers? | |
| Inner Setting | ||
|
System Level Implications: Barriers: Staffing problems Facilitators: Support for LCS from administrative and leadership team Provider/clinic Level Implications: Barriers: Insurance, adequate staffing, and time requirements to perform shared decision-making. Deficiencies of the electronic health record to find eligible participants also problematic. Facilitators: Giving personnel opportunities to inform LCS process. |
How will the infrastructure of your organization (social architecture, age, maturity, size, or physical layout) affect the implementation of the intervention? How will the infrastructure facilitate/hinder implementation of the intervention? | In your opinion, are there certain aspects of your LCS program structure or organization that helps or hinders adherence rates? |
| Are meetings, such as staff meetings, held regularly? | Does your LCS program hold regular meetings? Are problem areas and potential solutions discussed at these meetings? | |
| When you need to get something done or to solve a problem, who are your "go-to" people? | In your opinion, is your screening program leadership receptive to changes in workflow if identified from program personnel? | |
| Describe activities or initiatives that (appear to) have highest priority for you (for the organization)? | Describe activities or initiatives that appear to have the highest priority for your screening program. | |
| Do you expect to have sufficient resources to implement and administer the intervention? | Do you believe you have sufficient resources to address the adherence challenge? | |
| How do you expect to procure necessary resources? | What is the process to procure necessary support and resources for changes in workflow or quality improvement initiatives? | |
| Characteristics of Individuals | ||
|
System Level Implications: Barrier: Limited awareness and knowledge about LCS and adherence Facilitators: None Provider/clinic Level Implications: Barriers: Low knowledge and awareness among referring providers for shared decision-making and referrals. Patients also unaware of screening and are uncertain of positive results. Facilitators: High knowledge and awareness increases referrals |
What do you know about the intervention or its implementation? | In your opinion, are primary care/referring providers knowledgeable about LCS procedures/eligibility/guidelines? Are eligible participants becoming more knowledgeable? |
| Do you think the intervention will be effective in your setting? | Do you feel your adherence/tracking/reminder processes are efficient? What are the strengths? What are the weaknesses | |
| Process | ||
|
System Level Implications: Barriers: Process of setting up tracking mechanisms Facilitators: Having leadership or champion and being able to learn from other LCS programs Provider/clinic Level Implications: Barriers: Institutional policies may complicate clinic processes and availability of resources Facilitators: Buy-in from administration and having a navigator or coordinator to oversee the process |
Can you describe the plan for implementing the intervention? | Please tell me about the process your program uses for tracking annual and shorter term follow-up? How many reminders are sent? |
Step 2: Lung cancer screening navigator adherence semi-structured interview guide
| Introduction |
• Thank you for agreeing to participate in this interview. • The goal of this research project is to learn about how screening programs are addressing participant adherence, tracking, and reminders. • We are interested in learning more about processes and multi-level strategies used for adherence in your program. • Do you have questions before we begin the interview recording? • Can you please verbalize that you agree with audio recording of this interview? You are welcome to leave your camera off during the interview if that will make you feel more comfortable and, while we do not anticipate this, you are welcome to stop the interview at anytime if you start to feel uncomfortable. |
| Overview of Lung Cancer Screening Role and Perspectives |
1) To start, can you give me a quick overview of who does what in your screening program? a. Probe: How many personnel are involved with your program? b. Probe: Who completes referrals/SDM/tobacco cessation/tracking? c. Probe: What are the disciplinary backgrounds of the different positions of your program? 2) What is your current role within your lung cancer screening program and what are your day-to-day responsibilities? a. Probe: Please describe what your typical day looks like. b. Probe: How long have you been in this role? 3) Describe activities or initiatives that appear to have the highest priority for your screening program. a. Probe: Is there anything else you can say about why _________ is high priority for your program. b. Probe: Is this high priority something that was initiated by program leadership or more broadly at the health system level? 4) What do you think are the most important aspects of a successful lung cancer screening program? a. Probe: How do you define success for a lung cancer screening program? b. Probe: Does your program use specific metrics to track success? If so, what are those specifically? c. Probe: Does your definition of success differ from program leadership? |
| Adherence Processes and Available Resources |
5) Please tell me about the process your program uses for tracking annual and shorter term interval follow-up? a. Probe: How many reminders are sent? By whom? 6) If you could wave a magic wand, is there anything you would change about the current workflow of your LCS program overall? Specific to adherence? a. Probe: Do you feel your adherence/tracking/reminder processes are efficient? Why or why not? 7) Do you believe you have sufficient resources to address adherence and tracking? a. If no: what resources are you lacking? b. Probe: Do you believe cost is a prohibitive parameter for success of your program? Implementation procedures? Adherence? 8) In your opinion, are there certain aspects of your LCS program structure or organization that helps or hinders adherence rates? a. Probe: What are the strengths? What are the weaknesses? |
| Strategies Used to Facilitate Adherence |
9) Please tell me the role that electronic health records and/or tracking software has had in helping address adherence/tracking/reminders. a. Are there any capabilities you wish your EHR/tracking software had to help with adherence or reminders? 10) In your opinion, are primary care/referring providers knowledgeable about LCS procedures/eligibility/guidelines? Are eligible participants becoming more knowledgeable? a. Probe: How do you think knowledge has changed in the past 5 years? 11) What educational opportunities does your screening program offer to referring providers? How about eligible screening candidates? Are there any other concerted efforts made to educate the general public about lung cancer screening? Any efforts with community outreach and engagement around lung cancer screening in your catchment area? 12) To what extent were the needs and preferences of your LCS eligible population considered when deciding on your adherence procedures? Reminder materials? How about the needs and preferences of your ordering providers? a. Probe: How do you think this approach (engage/did not engage) has affected adherence? 13) Have you or your health system asked your LCS participants regarding their experiences with your tracking or reminder procedures? 14) Are you familiar with any barriers that your LCS participants’ experience that may keep them from being adherent to annual screening/follow-up? Does your program have any programs or solutions in place to help address these barriers? a. Probe: Can you describe a specific story? 15) Are there any additional ways or strategies your screening program uses to reach referring providers or participants to address adherence? a. Probe: Tell me more about __________. |
| Program Reflection and Quality Improvement |
16) Does your LCS program hold regular meetings? Are problem areas and potential solutions discussed at these meetings? 17) In your opinion, is your screening program leadership receptive to changes in workflow if identified from program personnel? a. Probe: Walk me through an example when changes in workflow were made. Who championed the process? 18) What is the process to procure necessary support and resources for changes in workflow or quality improvement initiatives? |
| Final question | 19) Is there anything else that you would like to tell me? Are there any questions I did not ask that you thought I should have? |
| Closing |
Thank you very much for answering questions about your role, screening program, and perspectives! Your input and time are greatly appreciated! Have a wonderful day |
Authors’ contributions
EAH: Conceptualization, Methodology, Visualization, Funding acquisition, Writing original draft. LCB: Conceptualization, Methodology, Visualization, Supervision, Writing – review & editing. JF and AC: Methodology, Writing – review & editing. All authors read and approved the final manuscript.
Funding
This work is supported by National Institutes of Health NCI F99/K00 Predoctoral to Postdoctoral Fellow Transition Award (K00CA264409, PI: Erin Hirsch). Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
This research was reviewed and approved by the Hackensack Meridian Institutional Review Board (Pro2023-0267).
Consent for publication
Not applicable.
Competing interests
EAH reports grants from NIH/National Cancer Institute and the Bristol MyersSquibb Foundation during the conduct of the study. JF reports institutional research support from Amgen, Bristol Myers Squibb Foundation, Disability Inclusion Network for Tobacco Control and Cancer Prevention, Patient Centered Oriented Research Institute, Thermo Fisher Scientific (all active). Institutional honoraria for serving on Patient Advocacy Advisory Boards for Bristol Myers Squibb, Merck, and Takeda. Personal honoraria for serving on Data Safety Management Board for research funded by the Patient Centered Oriented Research Institute. AC reports institutional research support for consulting fees from Daiichi Sankyo, Novartis, Seagen, and Genentech, and institutional honarium for advisory board participation from Glaxo Smith Kline. LC-B reports grants from the NIH/National Cancer Institute, Department of Defense, Gilead Sciences, and Merck Foundation during the conduct of the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Edwards N, Barker PM. The importance of context in implementation research. J Acquir Immune Defic Syndr. 2014;67(Suppl 2):S157–62. 10.1097/QAI.0000000000000322. [DOI] [PubMed] [Google Scholar]
- 2.May CR, Johnson M, Finch T. Implementation, context and complexity. Implement Sci. 2016;11(1):141. 10.1186/s13012-016-0506-3. Published 2016 Oct 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agency for Healthcare and Research. Prevention & chronic care program, improving primary care. Contextual factors: the importance of considering and reporting on context in research on the patient-centered medical home. AHRQ Publication No. 13–0045-EF. 2013. Available at: https://www.ahrq.gov/sites/default/files/wysiwyg/ncepcr/tools/PCMH/contextual-factors.pdf . Accessed 17 Apr 2024.
- 4.Nilsen P, Bernhardsson S. Context matters in implementation science: a scoping review of determinant frameworks that describe contextual determinants for implementation outcomes. BMC Health Serv Res. 2019;19(1):189. 10.1186/s12913-019-4015-3. Published 2019 Mar 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers L, De Brún A, McAuliffe E. Defining and assessing context in healthcare implementation studies: a systematic review. BMC Health Serv Res. 2020;20(1):591. 10.1186/s12913-020-05212-7. Published 2020 Jun 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ovretveit JC, Shekelle PG, Dy SM, et al. How does context affect interventions to improve patient safety? An assessment of evidence from studies of five patient safety practices and proposals for research. BMJ Qual Saf. 2011;20(7):604–10. 10.1136/bmjqs.2010.047035. [DOI] [PubMed] [Google Scholar]
- 7.National Cancer Institute. Surveillance, epidemiology and end results program. Cancer stat stats: Lung and Bronchus Cancer. Available at: https://seer.cancer.gov/statfacts/html/lungb.html. Accessed 17 Apr 2024.
- 8.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 9.US Preventive Services Task Force. Final recommendation statement. Lung cancer: screening. 2013. Available at: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening-december-2013. Accessed 17 Apr 2024.
- 10.National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382(6):503–13. 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 12.Special section: lung cancer. ACS cancer facts and figures 2023. 2023. Available at: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cff-special-section-lung-cancer.pdf. Accessed 17 Apr 2024.
- 13.US Preventive Services Task Force. Final recommendation statement. Lung cancer: screening. 2021. Available at: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening#fullrecommendationstart. Accessed 17 Apr 2024.
- 14.Sakoda LC, Henderson LM, Rivera MP. Adherence to lung cancer screening: what exactly are we talking about? Ann Am Thorac Soc. 2021;18(12):1951–2. 10.1513/AnnalsATS.202106-724VP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American College of Radiology Committee on Lung-RADS®. Lung-RADS assessment categories 2022. Available at https://www.acr.org/-/media/ACR/Files/RADS/Lung-RADS/Lung-RADS-2022.pdf. Accessed 4 Mar 2024.
- 16.Yousaf-Khan U, van der Aalst C, de Jong PA, et al. Final screening round of the NELSON lung cancer screening trial: the effect of a 2.5-year screening interval. Thorax. 2017;72(1):48–56. 10.1136/thoraxjnl-2016-208655. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Olivo MA, Maki KG, Choi NJ, et al. Patient adherence to screening for lung cancer in the US: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(11):e2025102. 10.1001/jamanetworkopen.2020.25102. Published 2020 Nov 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin Y, Fu M, Ding R, et al. Patient adherence to lung CT screening reporting & data system-recommended screening intervals in the United States: a systematic review and meta-analysis. J Thorac Oncol. 2022;17(1):38–55. 10.1016/j.jtho.2021.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silvestri GA, Goldman L, Tanner NT, et al. Outcomes from more than 1 million people screened for lung cancer with low-dose CT imaging. Chest. 2023;164(1):241–51. 10.1016/j.chest.2023.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rivera MP, Durham DD, Long JM, et al. Receipt of recommended follow-up care after a positive lung cancer screening examination. JAMA Netw Open. 2022;5(11):e2240403. 10.1001/jamanetworkopen.2022.40403. Published 2022 Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barta JA, Shusted CS, Ruane B, et al. Racial differences in lung cancer screening beliefs and screening adherence. Clin Lung Cancer. 2021;22(6):570–8. 10.1016/j.cllc.2021.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Kunitomo Y, Bade B, Gunderson CG, et al. Racial differences in adherence to lung cancer screening follow-up: a systematic review and meta-analysis. Chest. 2022;161(1):266–75. 10.1016/j.chest.2021.07.2172. [DOI] [PubMed] [Google Scholar]
- 23.Smith HB, Ward R, Frazier C, Angotti J, Tanner NT. Guideline-recommended lung cancer screening adherence is superior with a centralized approach. Chest. 2022;161(3):818–25. 10.1016/j.chest.2021.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Sakoda LC, Rivera MP, Zhang J, et al. Patterns and factors associated with adherence to lung cancer screening in diverse practice settings. JAMA Netw Open. 2021;4(4):e218559. 10.1001/jamanetworkopen.2021.8559. Published 2021 Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spalluto LB, Lewis JA, LaBaze S, et al. Association of a lung screening program coordinator with adherence to annual CT lung screening at a Large Academic Institution. J Am Coll Radiol. 2020;17(2):208–15. 10.1016/j.jacr.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirsch EA, New ML, Brown SP, Barón AE, Malkoski SP. Patient reminders and longitudinal adherence to lung cancer screening in an academic setting. Ann Am Thorac Soc. 2019;16(10):1329–32. 10.1513/AnnalsATS.201902-152RL. [DOI] [PubMed] [Google Scholar]
- 27.Mortman KD, Devlin J, Giang B, Mortman R, Sparks AD, Napolitano MA. Patient adherence in an Academic Medical Center’s low-dose computed tomography screening program. Am J Clin Oncol. 2021;44(6):264–8. 10.1097/COC.0000000000000817. [DOI] [PubMed] [Google Scholar]
- 28.Cattaneo SM 2nd, Meisenberg BR, Geronimo MCM, Bhandari B, Maxted JW, Brady-Copertino CJ. Lung cancer screening in the community setting. Ann Thorac Surg. 2018;105(6):1627–32. 10.1016/j.athoracsur.2018.01.075. [DOI] [PubMed] [Google Scholar]
- 29.Baron RC, Rimer BK, Breslow RA, et al. Client-directed interventions to increase community demand for breast, cervical, and colorectal cancer screening a systematic review. Am J Prev Med. 2008;35(1 Suppl):S34–55. 10.1016/j.amepre.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Sabatino SA, Lawrence B, Elder R, et al. Effectiveness of interventions to increase screening for breast, cervical, and colorectal cancers: nine updated systematic reviews for the guide to community preventive services. Am J Prev Med. 2012;43(1):97–118. 10.1016/j.amepre.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Bellinger C, Foley K, Genese F, Lampkin A, Kuperberg S. Factors affecting patient adherence to lung cancer screening. South Med J. 2020;113(11):564–7. 10.14423/SMJ.0000000000001167. [DOI] [PubMed] [Google Scholar]
- 32.Gagne SM, Fintelmann FJ, Flores EJ, et al. Evaluation of the informational content and readability of US lung cancer screening program websites. JAMA Netw Open. 2020;3(1):e1920431. 10.1001/jamanetworkopen.2019.20431. Published 2020 Jan 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haas K, Brillante C, Sharp L, et al. Lung cancer screening: assessment of health literacy and readability of online educational resources. BMC Public Health. 2018;18(1):1356. 10.1186/s12889-018-6278-8. Published 2018 Dec 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choo CW. The art of scanning the environment. In: Voros J, editor. Reframing environmental scanning: a reader on the art of scanning the environment. monograph series. No. 4. Hawthorn, Australia: Australian Foresight Institute, Swinburne University of Technology; 2003. p. 7–18. [Google Scholar]
- 35.Charlton P, Kean T, Liu RH, et al. Use of environmental scans in health services delivery research: a scoping review. BMJ Open. 2021;11(11):e050284. 10.1136/bmjopen-2021-050284. Published 2021 Nov 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rowel R, Moore ND, Nowrojee S, Memiah P, Bronner Y. The utility of the environmental scan for public health practice: lessons from an urban program to increase cancer screening. J Natl Med Assoc. 2005;97(4):527–34. [PMC free article] [PubMed] [Google Scholar]
- 37.Wilburn A, Vanderpool RC, Knight JR. Environmental scanning as a public health tool: Kentucky’s human papillomavirus vaccination project. Prev Chronic Dis. 2016;13:E109. 10.5888/pcd13.160165. Published 2016 Aug 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blanchard CM, Livet M. Ensuring intervention success: assessing fit as an overlooked step of the implementation process. Pharm Pract (Granada). 2020;18(4):2235. 10.18549/PharmPract.2020.4.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bero LA, Grilli R, Grimshaw JM, Harvey E, Oxman AD. Closing the gap between research and practice: an overview of systematic reviews of interventions to promote the implementation of research findings. The Cochrane Effective Practice and Organization of Care Review Group. BMJ. 1998;317(7156):465–8. 10.1136/bmj.317.7156.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Creswell J, Plano C. Choosing a mixed methods design. In: Designing and conducting mixed methods research. Thousand Oaks, CA: Sage Publications; 2011. p. 53–106. [Google Scholar]
- 41.Sharma A, Minh Duc NT, Luu Lam Thang T, Nam NH, Ng SJ, Abbas KS, Huy NT, Marušić A, Paul CL, Kwok J, Karbwang J, de Waure C, Drummond FJ, Kizawa Y, Taal E, Vermeulen J, Lee GHM, Gyedu A, To KG, Verra ML, Jacqz-Aigrain ÉM, Leclercq WKG, Salminen ST, Sherbourne CD, Mintzes B, Lozano S, Tran US, Matsui M, Karamouzian M. A consensus-based Checklist for Reporting of Survey Studies (CROSS). J Gen Intern Med. 2021;36(10):3179–87. 10.1007/s11606-021-06737-1. Epub 2021 Apr 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–57. [DOI] [PubMed] [Google Scholar]
- 43.Potthoff S, Finch T, Bührmann L, et al. Towards an Implementation-STakeholder Engagement Model (I-STEM) for improving health and social care services. Health Expect. 2023;26(5):1997–2012. 10.1111/hex.13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Academy of Engineering (US) and Institute of Medicine (US) Committee on Engineering and the Health Care System; Reid PP, Compton WD, Grossman JH, et al., editors. Building a better delivery system: a new engineering/health care partnership. Washington (DC): National Academies Press (US); 2005. 2, A framework for a systems approach to health care delivery. Available from: https://www.ncbi.nlm.nih.gov/books/NBK22878/. [PubMed]
- 45.Rihoux B, Ragin CC. Configurational comparative methods: qualiative comparative analysis (QCA) and related techniques. Thousand Oaks, CA: Sage; 2009. [Google Scholar]
- 46.Cragun D, Pal T, Vadaparampil ST, Baldwin J, Hampel H, DeBate RD. Qualitative comparative analysis: a hybrid method for identifying factors associated with program effectiveness. J Mix Methods Res. 2016;10(3):251–72. 10.1177/1558689815572023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanckel B, Petticrew M, Thomas J, Green J. The use of Qualitative Comparative Analysis (QCA) to address causality in complex systems: a systematic review of research on public health interventions. BMC Public Health. 2021;21(1):877. 10.1186/s12889-021-10926-2. Published 2021 May 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.GO2 Foundation for Lung Cancer. Risk & early detection. Screening Centers. Available at: https://go2.org/risk-early-detection/screening-centers/. Accessed on June 26, 2024.
- 49.Dillman DA, Smith JD, Christian LM. Internet, Phone, Mail, and Mixed-Mode Surveys: The Tailored Design Method. 4th ed. Hoboken, NJ, USA: Wiley; 2014. [Google Scholar]
- 50.Blanchard J, Rhoades D, Nagykaldi Z, et al. Identifying Priorities and Strategies for Improving Colorectal Cancer Screening in Tribal Clinics. Cancer Control. 2022;29:10732748221132516. 10.1177/10732748221132516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elkin EB, Snow JG, Leoce NM, Atoria CL, Schrag D. Mammography capacity and appointment wait times: barriers to breast cancer screening. Cancer Causes Control. 2012;23(1):45–50. 10.1007/s10552-011-9853-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Collie-Akers VL, Warrick C, Zhu L, Granado M, Ingram K. Assessment of characteristics of capacity among breast cancer screening facilities. J Community Health. 2012;37(3):626–31. 10.1007/s10900-011-9493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.D’Orsi C, Tu SP, Nakano C, et al. Current realities of delivering mammography services in the community: do challenges with staffing and scheduling exist? Radiology. 2005;235(2):391–5. 10.1148/radiol.2352040132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown ML, Klabunde CN, Mysliwiec P. Current capacity for endoscopic colorectal cancer screening in the United States: data from the National Cancer Institute Survey of Colorectal Cancer Screening Practices. Am J Med. 2003;115(2):129–33. 10.1016/s0002-9343(03)00297-3. [DOI] [PubMed] [Google Scholar]
- 55.Byrne MM, Hirsch EA, Hoover K, McCoy J, Blair C, Futrell M, Roy UB, Studts JL, Developing a conceptual framework for a person-centered approach to improving adherence and outcomes in lung cancer screening: the engaged approach to lung cancer screening (A Brief Report). JTO Clin Res Rep. 2024 (In press). 10.1016/j.jtocrr.2024.100728.
- 56.Clark SD, Reuland DS, Enyioha C, Jonas DE. Assessment of Lung Cancer Screening Program Websites. JAMA Intern Med. 2020;180(6):824–30. 10.1001/jamainternmed.2020.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dodd RH, Sharman AR, McGregor D, et al. Education messages and strategies to inform the public, potential screening candidates and healthcare providers about lung cancer screening: a systematic review. Prev Med. 2023;169: 107459. 10.1016/j.ypmed.2023.107459. [DOI] [PubMed] [Google Scholar]
- 58.Kincaid JP, Fishburne RP Jr, Rogers RL, Chissom BS. Derivation of new readability formulas (Automated Readability Index, Fog Count and Flesch Reading Ease Formula) for Navy Enlisted Personnel. Millington, TN: Institute for Simulation and Training, University of Central Florida; 1975. https://stars.library.ucf.edu/istlibrary/56. [Google Scholar]
- 59.McLaughlin GH. SMOG grading: a new readability formula. J Read. 1969;12(8):639–46. [Google Scholar]
- 60.Gunning R. The technique of clear writing. New York, NY: McGraw-Hill; 1952. [Google Scholar]
- 61.Hintze JL. PASS 11 User's Guide I. NCSS, LLC. Kaysville, UT, USA: NCSS, LLC; 2011. URL: https://www.ncss.com/wp-content/uploads/2012/09/PASS11UG1.pdf
- 62.SAS® Help Center. SAS® IT Resource Management 3.11. Administrator’s guide. Duplicate-data checking macros. Last updated September 14, 2023. Available at: https://documentation.sas.com/doc/en/itrmcdc/3.11/itrmxag/p1vokq23hmgn1en1ju1twoqyyl0p.htm. Accessed on September 25, 2024.
- 63.Kang H. The prevention and handling of the missing data. Korean J Anesthesiol. 2013;64(5):402–6. 10.4097/kjae.2013.64.5.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Willis GB. Cognitive interviewing: a tool for improving questionnaire design. Thousand Oaks: Sage Publications; 2004.
- 65.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;7(4):50. [DOI] [PMC free article] [PubMed]
- 66.Sedani AE, Davis OC, Clifton SC, Campbell JE, Chou AF. Facilitators and barriers to implementation of lung cancer screening: a framework-driven systematic review. J Natl Cancer Inst. 2022;114(11):1449–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kirk MA, Kelley C, Yankey N, Birken SA, Abadie B, Damschroder L. A systematic review of the use of the consolidated framework for implementation research. Implement Sci. 2016;17(11):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Keith RE, Crosson JC, O’Malley AS, Cromp D, Taylor EF. Using the Consolidated Framework for Implementation Research (CFIR) to produce actionable findings: a rapid-cycle evaluation approach to improving implementation. Implement Sci. 2017;12(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kiger ME, Varpio L. Thematic analysis of qualitative data: AMEE Guide No. 131. Med Teach. 2020;42(8):846–54. [DOI] [PubMed] [Google Scholar]
- 70.Ragin C. Studying cases as configurations. In: Ragin C, editor. Fuzzy-set social science. Chicago: University of Chicago Press; 2000. p. 64–87. [Google Scholar]
- 71.Núñez ER, Caverly TJ, Zhang S, et al. Adherence to follow-up testing recommendations in US veterans screened for lung cancer, 2015–2019. JAMA Netw Open. 2021;4(7):e2116233. 10.1001/jamanetworkopen.2021.16233. Published 2021 Jul 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seastedt KP, Luca MJ, Antevil JL, et al. Patient motivations for non-adherence to lung cancer screening in a military population. J Thorac Dis. 2020;12(10):5916–24. 10.21037/jtd-20-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goodrich DE, Miake-Lye I, Braganza MZ, et al. The QUERI roadmap for implementation and quality improvement. Washington (DC): Department of Veterans Affairs (US); 2020. Sustainment. Available from: https://www.ncbi.nlm.nih.gov/books/NBK566213/. [PubMed]
- 74.O’Leary K, Tanghe D, Pratt W, Ralston J. Collaborative health reminders and notifications: insights from prototypes. AMIA Annu Symp Proc. 2018;2018:837–46 Published 2018 Dec 5. [PMC free article] [PubMed] [Google Scholar]
- 75.Teo AR, Metcalf EE, Strange W, et al. Enhancing usability of appointment reminders: qualitative interviews of patients receiving care in the veterans health administration. J Gen Intern Med. 2021;36(1):121–8. 10.1007/s11606-020-06183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.AHRQ Health literacy: https://www.ahrq.gov/health-literacy/improve/precautions/tool11.html
- 77.Shahid R, Shoker M, Chu LM, Frehlick R, Ward H, Pahwa P. Impact of low health literacy on patients’ health outcomes: a multicenter cohort study. BMC Health Serv Res. 2022;22(1):1148. 10.1186/s12913-022-08527-9. Published 2022 Sep 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Davis TC, Arnold CL. Health Literacy Research in Rural Areas. Stud Health Technol Inform. 2020;269:241–7. 10.3233/SHTI200038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rikard RV, Thompson MS, McKinney J, Beauchamp A. Examining health literacy disparities in the United States: a third look at the National Assessment of Adult Literacy (NAAL). BMC Public Health. 2016;16(1):975. 10.1186/s12889-016-3621-9. Published 2016 Sep 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.