Abstract
This study investigated the physicochemical, low glycemic index, and mineral uptake of functional noodles added with varying concentrations (0–10 %) of Lentinus edodes (also known as shiitake) powder. Noodles with 4 % mushroom powder (L3) exhibited comparable sensory attributes in comparison with control noodles. The L3 showed significantly higher protein (1.92 ± 0.035 %), decreased carbohydrate content, improved cooking characteristics. The L3 exhibited darker color with lower hardness, adhesiveness, higher mineral content and bioavailability of iron (59.22 ± 0.49 %). As well, L3 showed a significantly higher mineral transport, retention, and uptake of minerals followed by increased ferritin content (29.17 ± 0.52 ng/mg cell protein). Mushroom powder incorporation in noodles significantly decreased the area under the curve of reducing sugar release correlating with lower glycemic index and thus a potential impact on blood sugar levels. This study illustrates the efficacy of mushroom powder as a functional component in noodles, offering both augmented nutritional advantages and increased glycemic regulation.
Keywords: Lentinus edodes, Mushroom, Noodles, Nutritional properties, Glycemic index, Mineral bioavailability, shiitake
Highlights
-
•
Functional noodles were prepared by adding Lentinus edodes mushroom powder.
-
•
Enhanced mineral content and bioavailability in mushroom-added noodles were observed.
-
•
L3 noodles demonstrated significant potential in modulating carbohydrate digestion in vitro
-
•
Integration of mushroom powder improved noodle texture and cooking quality.
1. Introduction
The increasing demand for functional foods has prompted extensive research on the addition of active ingredients to tradiitional food products with bioactive compounds to improve their nutritional and health benefits (Vlaicu et al., 2023). Among various functional foods, noodles stand out as a staple consumed widely across different cultures, making them an ideal product for dietary intake of essential nutrients (Rawat et al., 2023). The traditional noodles, however, are typically formulated using refined wheat flour which possesses a high glycemic index (GI) and significant nutritional value beyond carbohydrates. Due to these reasons, there is a need for modification in noodle formulations to create healthier alternatives that contribute to disease prevention, particularly in the management of conditions such as diabetes, cardiovascular diseases, and mineral deficiencies (Cui et al., 2024). A promising method for achieving this is the incorporation of edible mushroom powder into noodle formulations. Mushrooms possess notable culinary and medicinal properties, being rich in bioactive compounds including polysaccharides, dietary fibers, vitamins, minerals, and essential amino acids. (Łysakowska et al., 2023) Among different edible mushrooms, Lentinus edodes has gained attention for its wide-ranging health benefits, which are attributed to its unique composition of bioactive compounds. These mushrooms contain high levels of dietary fibers, such as β-glucans, which have been shown to exert positive effects on cholesterol levels and blood sugar regulation (Singh & Bhardwaj, 2023). Moreover, the mushroom is a rich source of micronutrients which play important roles in cellular processes such as antioxidant activity, and immune function. In addition to beta glucans moderate amount of proteins also contribute to lowering the glycemic index by slowing the digestion process and reducing the speed at which glucose enters the bloodstream (Chakraborty et al., 2023). All these characteristics enable shiitake mushrooms as an ideal source for the formulation of noodles which aim to reduce the glycemic index and enhance overall nutritional quality. Additionally, the incorporation of mushroom powder into noodles has the potential to increase the physiochemical properties of the product, including water absorption capacity, texture, and cooking stability, which are important factors in consumer acceptability (Shams et al., 2023). The incorporation of Lentinus edodes into noodles also significantly increases the mineral uptake as the mushroom is a good source of minerals such as iron, zinc, and calcium and also contains compounds that can enhance their bioavailability (Haftek, Abdayem and Guyonnet-Debersac, 2022, Hu et al., 2024). This study is aimed to enhance the mineral content and absorption in noodles, contributing to the development of functional foods that promote cellular health and overall well-being. In addition to their nutritional benefits, shiitake mushrooms have been associated with various health-promoting properties, including anti-inflammatory, antimicrobial, and cholesterol-lowering effects. These properties are attributed to the diverse array of bioactive compounds present in mushrooms, such as polysaccharides, terpenoids, sterols, and polyphenols (Rowaiye et al., 2022). The incorporation of shiitake mushroom powder to noodles presents a comprehensive method for enhancing health. The anti-inflammatory properties of beta-glucans may mitigate chronic inflammation, a prevalent underlying factor in numerous chronic diseases (Amaral et al., 2024). Similarly, the antimicrobial properties of shiitake mushrooms can enhance gut health by modulating the gut microbiota, thereby supporting overall digestive health. The innovation of functional noodles with Lentinus edodes mushroom powder also aligns with the growing interest in sustainable and plant-based diets (Singla et al., 2024). Mushrooms are considered an environmentally sustainable source of nutrition due to their low carbon footprint and efficient use of resources. The incorporation of mushroom powder into noodles facilitates the development of a sustainable food product that satisfies consumer nutritional requirements and addresses environmental issues (Jayaraman et al., 2024). This approach is particularly relevant in the context of global efforts to promote sustainable food systems and reduce the environmental impact of food production. Moreover, the development of functional noodles with enhanced nutritional profiles can have significant implications for public health. For instance, in regions where iron deficiency anemia is prevalent, functional noodles can provide a practical and accessible means of improving iron intake (Chaudhary et al., 2024). Similarly, in populations at risk of osteoporosis, enhanced calcium intake through functional noodles can support bone health (Biver et al., 2023) By targeting widely consumed food products, it is possible to achieve a broader impact on public health and contribute to the prevention and management of various nutritional deficiencies and chronic diseases. The unique flavor profile of shiitake mushrooms can enhance the sensory appeal of the noodles, which may lead to greater consumer acceptance (Sangeeta et al., 2024). The umami flavor of shiitake mushrooms is known to increase the overall taste of food, making it more palatable. This is especially advantageous in the development of functional foods that are both nutritious and enjoyable. The combination of enhanced nutritional value and sensory acceptance can, therefore, contribute to the overall success of functional noodle products in the market (Du et al., 2024). The production of functional noodles through the inclusion of Lentinus edodes mushroom powder offers an appealing approach for improving the nutritional and health benefits of a commonly consumed food. The rich nutritional profile and bioactive compounds of shiitake mushrooms can significantly improve the physicochemical attributes of noodles, enhance mineral uptake at the cellular level, and offer various health benefits. This study employed CaCo-2 cells, which are derived from human colon adenocarcinoma, and are commonly utilized for research on nutritional absorption and transport. When cultured, these cells can differentiate to form a monolayer of enterocyte-like cells. These cells contain microvilli similar to those found in small intestinal epithelial cells. This characteristic allows for the absorption of nutrients and minerals (Zhong and Kopec, 2024). Moreover, these cells can express the various nutrient transports and enzymes similar to those found in the human intestinal region that are essential for studying the mechanism of mineral uptake. The study provide insights into the development of innovative food products that support health and well-being. Functional noodles may be produced by harnessing the nutritional power of shiitake mushrooms, satisfying the demands of health-conscious customers and tackling the nutritional challenges faced by modern society.
2. Materials and methods
2.1. Materials and chemicals
Dried fruiting bodies of Lentinus edodes were sourced from the Directorate of the Mushroom Research Center, Solan, Himachal Pradesh. Basic noodle ingredients such as wheat flour, salt, carboxymethyl cellulose (CMC), and corn starch were purchased from local markets. All chemicals including concentrated sulfuric acid (H2SO4), boric acid, sodium hydroxide (NaOH), hydrochloric acid (HCl), and petroleum ether, were of analytical grade.
2.2. Preparation and optimization of mushroom added noodles
The preparation and formulation of mushroom-added noodles was done using the method described by Ng et al., 2022. In brief, five formulations of noodles (L2, L3, L4, L5, L6) were prepared by incorporating mushroom powder at concentrations of 2 g /100 g (2 %) wheat flour 4 g /100 g (4 %) wheat flour, 6 g /100 g (6 %) wheat flour 8 g/100 g (8 %) and 10 g /100 g (10 %) and noodle without mushroom powder (L1) was taken as control. To each formulation, a constant amount of salt (1.65 g), carboxymethylcellulose (CMC) (0.5 g), and corn starch (1 g) were added. All ingredients were thoroughly mixed, and water was gradually added to achieve a consistent dough texture. The dough was then kneaded until smooth and elastic, followed by a resting period of 15 min to allow for proper hydration and gluten relaxation. After resting, the dough was processed into noodles using a KENT noodles and pasta maker machine, ensuring uniform thickness and width. The freshly formed noodles were air dried at 60 °C for 9 h. Once dried, the noodles were cooled to room temperature and packaged in airtight plastic bags to maintain their quality. The packaged noodles were stored in a dry place for subsequent analysis.
2.3. Sensory analysis for the selection of noodles
The sensory analysis of the formulated mushroom-added noodles was conducted following the method described by Sushma et al. (2023). A panel of 30 trained panelists carried out the sensory evaluation, focusing on the attributes of color, flavor, texture, and overall acceptability of the noodles. The evaluation employed a nine-point hedonic scale, where a score of 1 represented ‘dislike extremely’ and a score of 9 indicated ‘like extremely’. Ethical permission for the study was secured to ensure compliance with relevant ethical standards and guidelines for sensory testing involving human participants. The panelists were selected based on their experience and training in sensory evaluation to ensure the reliability and accuracy of the results. All panelists signed the informed consent form detailing the procedure and objectives of the study. The review and approval for sensory analysis were given by the institutional ethical committee (reference number LPU/CA/026/03/13092). Each panelist individually evaluated the noodles under controlled conditions, and the collected data was utilized to ascertain the most favored formulation based on the measured sensory qualities.
2.4. Nutritional value analysis of noodles
2.4.1. Moisture content
The nutritional profile of noodles incorporated with L. edodes mushroom powder was determined by following the AOAC, 2000 method. Herein, 2 g of each control noodle sample and the 4 % mushroom-containing sample were evaluated for moisture content analysis. The samples were placed in petri dishes and dried at 100 °C for 2 h in a hot air oven. The observed weight of samples was recorded once the weight of each sample was constant. All experiments are performed in triplicates. The moisture content (%) of the samples was calculated by following eq. 1:
| (1) |
here, IW=Initial weight, FW = final weight.
2.4.2. Protein content
Protein content of the noodles samples was carried out using the Kjeldhal method. In brief, 1 g of each control and 4 % mushroom powdered noodles samples were put in digestion tubes containing 20 mL of concentrated H2SO4 burned for 2 h at 400 °C. The sample was allowed to cool and 100 mL of distilled water was added to each test tube. The distillation of the sample was done 40 % of NaOH was added during this process. The distillation process was followed by titration. Herein, the sample was taken in a flask containing ammonia gas. To the sample, 2 % boric acid was added and titrated against 0.1 N HCl until it reached a pink color. The process followed the described by Amerikanou et al., 2023. The yield of Nitrogen was calculated by following eq. 2, and the protein content (%) was calculated by using following eq. 3:
| (2) |
| (3) |
here, N = Nitrogen, TV = Titer value, BV = Blank value, W = weight of the sample.
2.4.3. Ash content determination
The determination of ash value was done according to AOAC, 2000. In brief, the weight of the clean-dried crucible with 3 g of noodles samples was noted. The sample containing the crucible was put in the hot air oven at 700 °C for 2 h, and after 2 h final weight of the sample was observed calculation was done by following eq. 4:
| (4) |
here, IW = Initial weight, FW = Final weight.
2.4.4. Fat content
Fat content in the noodle samples was determined using a Soxtherm extraction method following Zakaria et al., 2022. In brief, a thimble was loaded with 3 g of noodle powdered sample and placed within the Soxhlet apparatus and a blank dried flask containing 250 mL of petroleum ether. The extraction process ran for 4 h. After 4 h the flask containing the extracted fat residue was then dried in a hot air oven at 100 °C for 45 min and after cooling the flask, the final weight of the flask with fat residue was measured. The fat (%) content was calculated by using the following eq. 5:
| (5) |
2.4.5. Fiber content
The fiber content was determined using the method given by AOAC, 2000. In detail, moisture and fat-free noodles powder sample (1 g) was weighed and put into fiber bags. A glass spacer was added to the bags before the sample carousel and put into a glass container with 500 mL of 1.25 % dilute H2SO4, and it was heated for 30 min. The bags were then boiled in 500 mL of distilled water for 30 min followed by the addition of 500 mL of 1.25 % NaOH solution and heated for another 30 min. Subsequently, 500 mL of distilled was added and boiled for an additional 30 min, after transferring the residue to a pre-weighed clean crucible and dried using a hot air oven at 110 °C for 3 h. Finally, the crucible was incinerated in a muffle furnace at 600 °C for 2 h and weighed once more. The fiber content was calculated by using the following eq. 6:
| (6) |
2.4.6. Determination of the carbohydrate and energy content
Carbohydrate concentration and the energy content of the noodles sample were calculated by a general method, followed by Zakaria et al., 2022 and Nordiana et al., 2019 The calculation was followed using the eq. (7), (8):
| (7) |
| (8) |
2.5. Color
The color value of the noodles was detected using the method by Baltacıoğlu et al., 2021. The L*, a*, and b* values of the noodles were measured where L∗ indicates lightness/darkness, a∗ indicates redness/greenness, and b∗ indicates yellowness/blueness values.
2.6. Textural properties analysis
The texture property was analyzed according to Wang, Brennan, et al., 2021; Zhang et al., 2022 methods with slightly modifications. Herein, 20 g of each sample control and 4 % mushroom-incorporated noodles were boiled for 6 min, and 5 min respectively. After boiling the noodles were dipped in cold water for around 40 s and put on filter paper to remove the excess water of surface. Then samples were set on the test plate of the texture analyzer (TA-HDplusC, Stable Micro Systems), and used P/25 probe for the analysis of the texture. The whole process was performed eight repeated times.
2.7. Determination of cooking characteristics of noodles
2.7.1. Cooking time and water absorption
The perfect cooking time and the water absorption properties of the noodles were performed followed by Shams et al., 2023 and Parvin et al., 2020, respectively. In brief, 3 g of noodles sample was kept in a beaker containing 45 mL of distilled water and boiled. The optimum cooking time of the noodles was recorded. Further, to assess the water absorption capacity of noodles, 3 g of noodles were kept in 50 mL of distilled water for 8–10 h. The experiment was carried out in triplicate. The water absorption (%) was calculated by using the following eq. 9:
| (9) |
here, FW = Final weight, IW = initial weight.
2.8. Fourier transform infrared spectroscopy (FTIR)
The functional properties of the control and the mushroom-incorporated noodles were examined by using FTIR following the method described by Arora et al., 2021. FTIR with Diamond Attenuated Total Reflectance and Pellet (Perkin Elmer, Spectrum Two, USA) was utilized to record the spectra. Both the noodles samples (8 mg) and the mushroom powder (8 mg) were placed on the mirror surface of the machine via air as its background and spectral range of 4000–400 cm−1, data was acquired in terms of transmittance using the Spectrum 10 software.
2.9. Mineral content estimation
The mineral content, including iron, zinc, calcium, copper, magnesium, iodine, and potassium of mushroom-incorporated noodles, was evaluated following the methodology described by Patil et al., 2024 The quantification of the concentration of minerals was done by using the Inductively coupled plasma optical emission spectroscopy (ICP-OES; Thermo Scientific, iCAP 7000 series, Germany) method. The configuration and calibration of the optical system of the ICP-OES spectrometer was done using the Intelligent Calibration and Logic (ICAL) method, as outlined by Spectro Analytical Instruments GmbH in 2012. A serial dilution of a multielement standard solution with deionized water was performed to produce a calibration curve, and the analysis of iron, zinc, calcium, copper, magnesium, sodium, and potassium was conducted by measuring at wavelengths of 238.204 nm, 213.856 nm, 396.847 nm, 324.754 nm, 285.213 nm, 589.592 nm, and 769.896 nm, respectively, utilizing radial plasma observation. To avoid precipitation, no flux or hydrochloric acid was introduced during calibration to simulate the sample matrix preparation by melting. A 20 mg/L solution of iron, zinc, and calcium was employed for initial calibration verification (ICV), adhering to an acceptance threshold of ± 10%, in accordance with the guidelines of Spectro Analytical Instruments GmbH and the U.S. Environmental Protection Agency (EPA). The control sample was analyzed after every ten samples to ensure the accuracy and reliability of the measurements.
2.9.1. In vitro mineral bioavailability of noodles
The in vitro bioavailability of iron, zinc, and calcium of mushroom incorporated noodles was analyzed using Kała et al., 2021 study. The study aimed efficiency of absorption and utilization of minerals by the human body. The experiment stimulated the process of gastrointestinal digestion and evaluation of cellular absorption was done by Caco-2 cell lines in a trans-well assay. These methods are employed to understand the bioavailability of the minerals and their potential nutritional benefits on human health.
2.9.2. Cellular mineral uptake using Caco-2 cell line model
The culturing of Caco-2 cells was done utilizing the methodology outlined by Lavanya et al., 2023. The growth medium composed of antibiotic solution (39 μg/mL Streptomycin, 25 μg/mL Amphotericin, and 100 μg/mL Penicillin), l-Glutamine (2 mM), heat-inactivated fetal bovine serum (10 %), and DMEM (1 %). The cells were incubated in a CO2 incubator (Thermo Fisher Scientific, Mumbai, India), at 35 °C with 96 % humidity and 4 % CO2 levels. The growth media was replaced with fresh one every day, and cells were washed five times with PBS (phosphate-buffered saline). Upon 90 % confluence of cells the cell passaging was performed for 7–8 days. The cells were further subcultured utilizing 0.05 % EDTA and 0.5 % trypsin. Caco-2 cells at the 39th passage were seeded at a density of 50,000 cells per well in six-well plates equipped with sterile polyester membranes (24 mm diameter and 0.4 μm pore size) for subsequent mineral absorption assays to facilitate the cellular transfer of iron, zinc, and calcium. Following an initial 4–5 days, the cells underwent an additional 10-day differentiation period before conducting the transepithelial cellular absorption assay.
2.9.3. Ferritin content
The accumulation of ferritin within the cells in the cell line was assessed by utilizing the Human Ferritin ELISA kit (Thermo Fisher Scientific, Mumbai, India). The cells were washed with PBS, dissociated using trypsin, and were collected. The dissociated cells were re-suspended in 2 mL of cell-grade water and lysed using a probe sonicator at 4 °C for 2 min, using 5-s pulses. The specific protocol assay was followed to measure ferritin content.
2.10. In vitro simulated intestinal digestion
The in vitro intestinal digestion was determined to measure the breakdown of carbohydrates to reducing sugars as per method followed by Wang, Tian, et al., 2021. In brief, the noodles (500 g) were cooked in 1 L of distilled water for 10–20 min and were cut to 2-5 mm size, and 3 g of cooked noodles were transferred to a plastic biopsy pot and stirred constantly for 15 min at 37 °C on a magnetic stirrer. The stomach digestion started by adding 800 μL HCl (1 M), and 1000 μL pepsin (10 %) solution by stirring constantly for 35 min. The small intestine phase digestion is represented by the addition of 0.1 M, sodium maleate (pH 6) and 350 U/mg 2.5 % pancreatin (5 mL) solution for 1 h 20 min. The mixture (2 mL) thus obtained was dissolved in 8 mL of ethanol after different intervals of time (20 min, 1 h, and 1 h 20 min) and kept at 4 °C. The analysis of reducing sugar content was carried out by a 3,5-dinitro salicylic acid method. The digest was centrifuged at 1000 rpm for 10 min and the supernatant was collected and stored at ˗20 °C for further analysis.
2.11. Total phenolic components
The total phenolic components were stated by the method followed by Wang et al., 2020. Herein, 400 μL of both L1 and L3 samples of noodles were mixed thoroughly in 2 mL Folin-Ciocalteu reagent (1 N) and 4 mL sodium carbonate (7.5 %). The mixture was then kept in dark condition for 30 min and then absorbance was measured at 760 nm by UV–Visible spectrophotometer. The calibration curve was plotted using different concentrations of standard gallic acid and total phenolic acid was calculated GAE/g.
2.12. Total flavonoid content
Albumin chloride assay was used to calculate the total flavonoid present in L1 and L3 noodle samples. In brief, 4 mL of both samples were dissolved in 5 % sodium nitrite (400 μL) and kept in a constant position for 5 min. After 5 min of incubation, the mixture was treated with 10 % aluminum chloride (400 μL) and dissolved properly using a vortex shaker. The reaction mixture was then kept undisturbed for 8 min and 4 mL NaOH (1 M) was added to it. The absorbance of the reaction mixture was then measured at 510 nm using a UV–visible spectrometer. The calibration curve was plotted using different concentrations of quercetin and the total flavonoid was calculated (Bains et al., 2021).
2.13. In vitro antioxidant activity
The in vitro antioxidant activity of mushroom-incorporated noodles was determined according to methods of Zakaria et al., 2022. Herein, a 250 μL sample of noodles was added in 2.50 mL of DPPH solution in a test tube and kept in the dark for 30 min. The change in the color of DPPH from purple to yellow was observed and absorbance was measured at 517 nm using UV-spectrophotometer. The percentage of free radical activity was calculated by following the equation
Here L3 is the absorption of the mushroom incorporated noodle sample, L absorbance of the DPPH solution (control).
2.14. Statistical analysis
The standard error of the mean was computed using the Data Analysis Toolpak in Microsoft Excel 2021 (Microsoft Corp., Redmond, WA). Statistical significance and the determination of differences between groups were analyzed through one-way and two-way analysis of variance (ANOVA).
3. Results and discussion
3.1. Sensory analysis of mushroom-incorporated noodles
The sensory analysis of different concentrations of mushroom-incorporated noodles is presented in Table 1. Statistically in terms of taste and aroma, texture and mouth feel as well as overall acceptability control the noodle samples L2 and L3 showed non-significant differences (p < 0.05). However, there was a significant difference (p < 0.05) between the control and both samples in terms of color and appearance. According to the results, both the control and the L3 mushroom sample exhibited the highest scores in overall acceptance (8.8 and 8.64, respectively), indicating good consumer acceptance compared to other concentrations. Based on the sensory analysis, the L3(4 %) noodle was ultimately recognized as a consumer-accepted product and selected for further analysis. The present study follows the study done by Parvin et al., 2020, who utilized P. ostreatus powder for the preparation of noodles in four varied concentrations 5 %, 8 %, and 10 % as well as 2 %, 5 %, 7 %, 8 %, and 10 % respectively. In their study, the noodles formulated using 5 % received the highest sensory score.
Table 1.
Sensory analysis of different concentrations of mushroom-incorporated noodles.
| Sample | Color and appearance | Taste and Aroma | Texture and mouthfeel | Overall acceptability |
|---|---|---|---|---|
| L1 (Control) | 8.91 ± 0.72⁎⁎⁎⁎ | 8.68 ± 0.68⁎⁎⁎ | 8.84 ± 0.45⁎⁎⁎ | 8.82 ± 0.56⁎⁎⁎ |
| L2(2 %) | 7.72 ± 0.37⁎⁎⁎ | 8.54 ± 0.57⁎⁎⁎ | 8.72 ± 0.42⁎⁎⁎ | 8.64 ± 0.48⁎⁎⁎ |
| L3(4 %) | 7.68 ± 0.45⁎⁎⁎ | 8.52 ± 0.42⁎⁎⁎ | 8.74 ± 0.37⁎⁎⁎ | 8.67 ± 0.39⁎⁎⁎ |
| L4(6 %) | 6.89 ± 0.36⁎⁎ | 7.22 ± 0.56⁎⁎ | 7.24 ± 0.45⁎⁎ | 7.08 ± 0.54⁎⁎ |
| L5(8 %) | 6.73 ± 0.35⁎⁎ | 6.64 ± 0.39⁎ | 6.55 ± 0.36⁎ | 6.54 ± 0.37⁎ |
| L6(10 %) | 5.51 ± 0.42⁎ | 6.25 ± 0.47⁎ | 6.42 ± 0.65⁎ | 6.48 ± 0.42⁎ |
Results are expressed as means (n = 3) ± standard deviation. The sign *, **, ***, and **** denote significantly differences within the column (p ≤ 0.05).
3.2. Nutritional profile analysis of Lentinus edodes formulated noodles
The nutritional profile analysis of formulated noodles was done and the results are shown in Table 2. In the present study it has been observed in comparison to L1 incorporated with 4 % mushrooms have significant (p < 0.05) higher content of fiber (0.60 ± 0.03 %), fat (0.30 ± 0.02 %), protein (1.92 ± 0.03 %), and ash (3.55 ± 1.38 %). However, mushroom-incorporated noodles have significantly lower (p < 0.05) carbohydrate (92.30 ± 0.58 %), moisture content (1.33 ± 0.28 %) and energy (379.58 ± 0.32 kcal) in comparison to control. The decrease in carbohydrate content is due to the presence of non-digestible carbohydrates such as starch, oligosaccharides, chitin, and β-glucans (Karimi et al., 2024). The present results are in accordance with the study carried out by Parvin et al., 2020, who formulated the noodles by incorporating P. ostreatus mushroom powder.
Table 2.
Nutritional profiling of control and mushroom-incorporated noodles.
| Nutrients | Control (L1) | L3(4 %) |
|---|---|---|
| Ash (%) | 1.66 ± 0.33⁎ | 3.55 ± 1.38⁎⁎ |
| Moisture (%) | 2.33 ± 0.76⁎⁎ | 1.33 ± 0.28⁎ |
| Protein (%) | 1.67 ± 0.01⁎ | 1.92 ± 0.03⁎⁎ |
| Fat(g) | 0.26 ± 0.01⁎ | 0.30 ± 0.02⁎ |
| Fiber(g) | 0.14 ± 0.02⁎ | 0.60 ± 0.03⁎⁎ |
| Carbohydrate (%) | 93.94 ± 0.32⁎⁎ | 92.30 ± 0.58⁎ |
| Energy (kcal) | 384.78 ± 0.17⁎⁎ | 379.58 ± 0.32⁎ |
Results are expressed as means (n = 3) ± standard deviation. The sign *, and **, denote significantly differences within a row (p ≤ 0.05).
3.3. Color
The noodles' color is an important factor in determining their quality and influences customers' decisions to purchase. Table 3 reveals the 4 % mushroom-incorporated noodles displayed a darker color (lower L value) and a shift towards yellow and red (higher a and b values) compared to the L1. The dark color of noodles depends upon the concentration of mushrooms used and the drying condition. The oxidation reaction along with the Millard reaction occurs between the proteins and carbohydrates during drying resulting in the darker color of the noodles (Bhatt and Gupta, 2023). The present results are similar to the results obtained by Wang, Brennan, et al., 2021, who observed an increase in the value of a*, and b* and a decrease in L* value upon the addition of Hericium erinaceus mushroom powder in noodles. Another study conducted by Nordiana et al., 2019, observed that Pleurotus sajor-caju incorporated pasta showed a decreased L* value and increased a*, and b* values compared to control pasta.
Table 3.
Color properties of control and mushroom-incorporated noodles.
| Color analysis | Control (L1) | L3 (4 %) |
|---|---|---|
| L* value | 55.46 ± 0.25 | 11.81 ± 0.10 |
| a* value | −1.81 ± 0.03 | 3.72 ± 0.06 |
| b* value | 15.19 ± 0.44 | 18.84 ± 0.31 |
3.4. Texture properties of mushroom-incorporated noodles sample
The textural properties of formulated noodles are shown in Table 4. In the present study, it has been observed that in comparison to L1 incorporated with 4 % mushroom powder have significantly (p < 0.05) less hardness and adhesiveness. The lowering in the value of hardness is due to the reduction in the gluten network strength of mushroom-incorporated noodles (Li et al., 2023). Furthermore, the lowering in the value of adhesiveness of formulated noodles is due to the presence of a high concentration of fibers that results in disruption of the protein-starch matrix (Zhang et al., 2022). The present results lined with the results obtained by Shams et al., 2023, who formulated noodles using button mushroom (Agaricus bisporus) powder and chickpea starch. Similarly, Nordiana et al., 2019, observed that Pleurotus sajor-caju powder incorporated pasta showed a lower value of hardness and adhesiveness compared to the L1.
Table 4.
Textural properties of control and mushroom-incorporated noodles.
| Texture Profile | Control (L1) | L3 (4 %) |
|---|---|---|
| Hardness(g) | 2171.19 ± 168.00 | 1484.02 ± 383.01 |
| Adhesiveness (g.sec) | −133.96 ± 5.62 | −107.47 ± 16.60 |
Results are expressed as means (n = 3) ± standard deviation.
3.5. Determination of cooking characteristics of noodles
The effect on cooking properties such as cooking time water absorption due to the incorporation of mushroom powder in noodles is represented in Table 5. In the present study, it has been observed that there was an increase in water absorption and a decrease in cooking time upon the addition of mushroom powder in wheat flour. The increase in water absorption is due to the presence of fibers that endorse the absorption of water and hold the network of starch proteins resulting in the swelling of starch granules (Ali et al., 2024). The present study aligns with the study done by Parvin et al., 2020, who observed similar cooking characteristic properties for noodles formulated using P. ostreatus mushroom powder. They observed Pleurotus ostreatus-formulated noodles required 5.3 min to cook, whereas control noodles required just 6.5 min. Similarly, a study by Nordiana et al. (2019) further observed that increasing the amount of Pleurotus sajor-caju powder in pasta samples led to progressively shorter cooking times. Shams et al., 2023, found that increasing the content of both mushroom powder Agaricus bisporus and chickpea starch in noodles led to faster cooking times and enhanced the water absorption capacity.
Table 5.
Cooking characteristics of control and mushroom-incorporated noodles.
| Cooking characteristics | Control (L1) | L3(4 %) |
|---|---|---|
| Cooking time (min) | 6.23 ± 0.06⁎⁎ | 4.70 ± 0.04⁎ |
| Water absorption (%) | 109.17 ± 0.37⁎ | 116.18 ± 0.17⁎⁎ |
Results are expressed as means (n = 3) ± standard deviation. The sign *, and **, denote significantly differences within a row (p ≤ 0.05).
3.6. FTIR spectroscopy of L. edodes powder formulated noodles
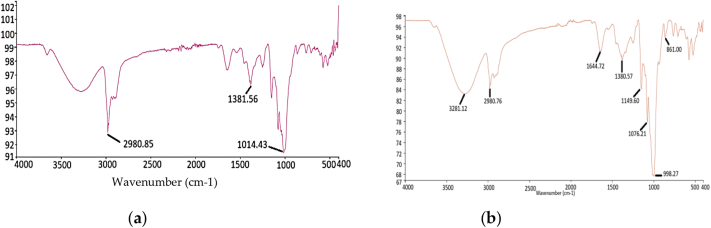
The FTIR spectroscopy was used to analyze the functional group of L1 and L3 (Fig. 1a, b). The results showed significant differences between the two types of noodles. The infrared spectrum analysis of the L1 showed three peaks at 2980.85 cm−1, 1381.56 cm−1, and 1014.43 cm−1, which are highly similar to those observed in the mushroom-formulated noodles (2980.76 cm−1, 1380.57 cm−1, 1076.21 cm−1, and 998.27 cm−1) indicating the presence of C—H stretching, CH3 group, and C—O stretching (Baltacıoğlu et al., 2021). However, the addition of L. edodes mushroom powder to the noodles introduces new functional groups, which can indicate the presence of specific compounds. The peak at 3281.12 cm−1 indicates the presence of O—H stretching common in β-glucans, phenolic compounds, and sugar or sugar alcohol such as mannitol. The peak of 1644.72 cm−1 indicates the presence of C O and NH stretching of proteins, and 861.00 cm−1 indicates the presence of the C—O group of polysaccharides that contributes to the dietary fibers (Zhang et al., 2022). The FTIR spectroscopy analysis, therefore, reveals that the integration of L. edodes mushroom powder into noodles results in significant functional group changes, introducing hydroxyl, carbonyl, amine, and polysaccharide-related groups. All these modifications potentially enhance the nutritional and functional properties of the noodles in comparison to control. The present results are in accordance with the results obtained by Shashikant et al., 2022; Baltacıoğlu et al., 2021.
Fig. 1.
FTIR spectra: (a) Control (L1) noodles; (b) L3 (4 %) mushroom-incorporated noodles.
3.7. Mineral analysis
The estimation of the mineral content of mushroom powder, L1, and L3 was performed and the results are represented in Table 6. In the present study it has been observed that in comparison to L1, and L3, mushroom powder revealed significantly higher (p < 0.05) iron (24.63 ± 0.34 mg/1000 g), zinc (7.96 ± 0.24 mg/1000 g), calcium (36.29 ± 0.98 mg/1000 g), copper (59.91 ± 0.47 mg/1000 g), magnesium (89.82 ± 0.72 mg/1000 g), sodium (49.98 ± 0.81 mg/1000 g) and potassium (4332.69 ± 23.97 mg/1000 g). However, mushroom powder noodles showed significantly higher (p < 0.05) iron (9.93 ± 0.35 mg/1000 g), zinc (6.43 ± 0.27 mg/1000 g), calcium (12.84 ± 0.87 mg/1000 g), copper (17.93 ± 0.56 mg/1000 g), magnesium (48.73 ± 0.67 mg/1000 g), sodium (23.73 ± 0.89 mg/1000 g) and potassium (361 ± 26.94 mg/1000 g) in comparison to L1. These findings, therefore, highlight that the incorporation of mushroom powder in noodles has potential health benefits such as bone health, neurological function, and metabolic processes. The study suggests that mushroom noodles can be a valuable addition to the diet thereby contributing uniquely to meet different mineral requirements and overall nutritional well-being. The present results lined with the study of Parvin et al., 2020, who prepared mushroom-fortified noodles and studied the quality improvement by comparing them with available local branded noodles.
Table 6.
Determination of mineral content present in mushroom powder, control noodles, and mushroom-incorporated noodles.
| Mineral content (ppm) | Mushroom powder (mg/ 1000 g) | Control (L1) (mg/ 1000 g) | L3(4 %) (mg/ 1000 g) |
|---|---|---|---|
| Iron | 24.63 ± 0.34⁎⁎⁎ | 6.63 ± 0.87⁎ | 9.93 ± 0.35⁎⁎ |
| Zinc | 7.96 ± 0.24⁎⁎⁎ | 2.96 ± 0.39⁎ | 6.43 ± 0.27⁎⁎ |
| Calcium | 36.29 ± 0.98⁎⁎⁎ | 9.83 ± 0.81⁎ | 12.84 ± 0.87⁎⁎ |
| Copper | 59.91 ± 0.47⁎⁎⁎ | 14.56 ± 0.62⁎ | 17.93 ± 0.56⁎⁎ |
| Magnesium | 89.82 ± 0.72⁎⁎⁎ | 34.73 ± 0.88⁎ | 48.73 ± 0.67⁎⁎ |
| Sodium | 49.98 ± 0.81⁎⁎⁎ | 18.92 ± 0.79⁎ | 23.73 ± 0.89⁎⁎ |
| Potassium | 4332.69 ± 23.97⁎⁎⁎ | 194.56 ± 28.89⁎ | 361 ± 26.94⁎⁎ |
Results are expressed as means (n = 3) ± standard deviation. The sign *, ** and *** denote significantly differences within a row, (p ≤ 0.05).
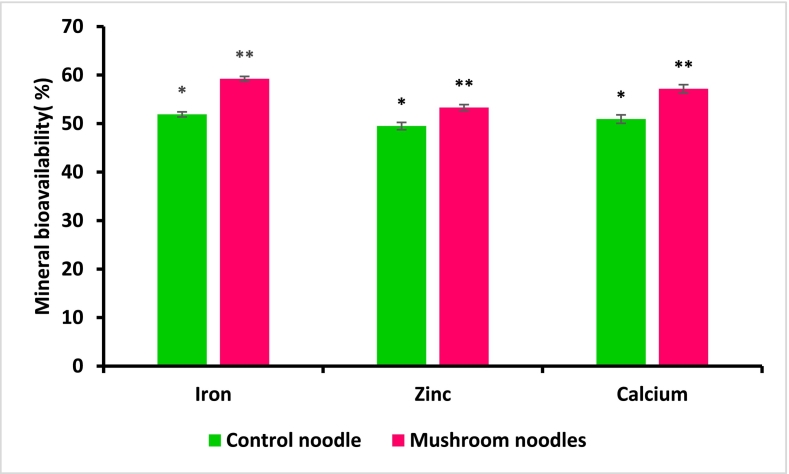
3.8. Mineral bioavailability
The results of the bioavailability of minerals such as iron, zinc, and calcium present in L3 are shown in Fig. 2 and it has been observed that in comparison to L1, L3 showed significantly high (p < 0.05) availability of iron (59.22 ± 0.49 %), zinc (53.29 ± 0.62 %) and calcium (57.16 ± 0.85 %). These findings suggest that mushroom powder incorporated mushrooms are a valuable source of essential minerals thereby making it beneficial for dietary mineral uptake.
Fig. 2.
In vitro mineral bioavailability of control noodles (L1) and mushroom-incorporated noodles (L3). The sign *, and ** denote differences within the column (p ≤ 0.05).
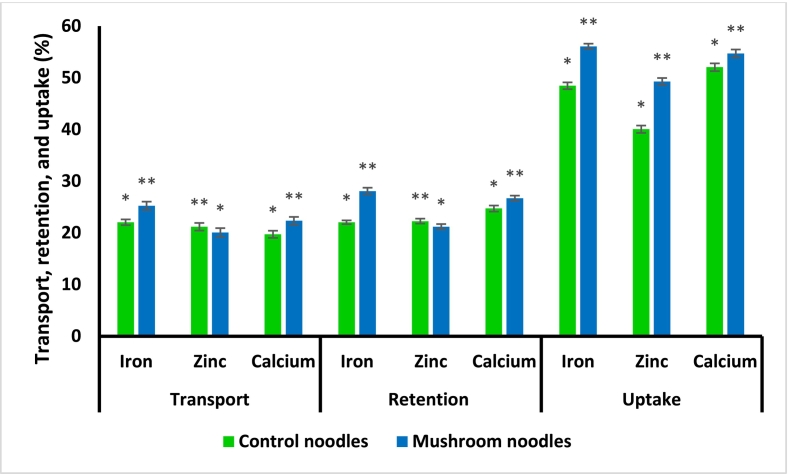
3.9. Mineral uptake by CaCO-2 cell lines
The digested samples obtained from stimulated gastrointestinal digestion were subjected to a trans-well assay to evaluate the cellular absorption of minerals. CaCO-2 cells in the apical chamber were treated with 50 μM concentration of iron, zinc, and calcium. In the present study, it has been observed that L3 in comparison to L1 showed a significantly high (p < 0.05) percentage of transport, retention, and uptake of iron (25.24 ± 0.81 %, 28.1 ± 0.64 %, 56.09 ± 0.52 %), followed by calcium (22.34 ± 0.76 %, 26.73 ± 0.49 %, 54.71 ± 0.77 %), and zinc (20.07 ± 0.85 %, 21.19 ± 0.52 %, 49.28 ± 0.68 %) respectively as shown in Fig. 3. The present results thus indicate that L3 can be more effective in enhancing all mineral bioavailability due to the superior retention capabilities and, therefore, can optimize the bioavailability of these essential minerals. The present results are in accordance with the study done by Kała et al., 2021, who determined the amount of potentially bioavailable phenolic compounds and elements present in the mycelial culture of three edible mushrooms namely Agaricus bisporous, Cantharellus cibarius, and Lentinula edodes.
Fig. 3.
In vitro transport, retention, and uptake of minerals by CaCo2 cell lines. The sign *, and ** denote significantly differences within the column (p ≤ 0.05).
3.9.1. Ferritin synthesis
The ferritin content was determined to evaluate the storage of iron in cells. The measurement of cellular iron uptake was measured as the ratio of ferritin to cell protein (ng ferritin/mg cell protein) and the results are represented in Table 7. The present study demonstrates that there was a significant increase (p < 0.05) in the synthesis of ferritin in CaCo-2 cells upon exposure to L3 and L1 in comparison to control media. L1 resulted in 23.68 ± 0.61 ng ferritin mg cell per protein, while L3 represented a significantly high synthesis of ferritin at 29.17 ± 0.52 ng ferritin mg cell per protein. Furthermore, the cell protein content was also significantly higher (p < 0.05) in cells treated with L3 136.38 ± 0.49 ng ferritin. mg cell protein-1. g sample−1 in comparison to those treated with L1 129.55 ± 0.56 ng ferritin. mg cell protein-1. g sample−1. Therefore, it has been concluded from the present study that L3 can effectively stimulate iron uptake and storage mechanisms. The present findings lined with the study done by Oyetayo et al., 2021, who evaluated the biological efficiency, nutrient contents, and antioxidant activity of Pleurotus pulmonarius enriched with zinc and iron.
Table 7.
In vitro cellular ferritin synthesis in Caco-2 cells.
| Ferritin synthesis in Caco2 cells |
||
|---|---|---|
| ng ferritin. mg cell protein−1 | ng ferritin. mg cell protein−1. g sample−1 | |
| Media | 2.51 ± 0.04 | – |
| Control (L1) | 23.68 ± 0.61⁎ | 129.55 ± 0.56⁎ |
| L3 (4 %) | 29.17 ± 0.52⁎⁎ | 136.38 ± 0.49⁎⁎ |
Results are expressed as means (n = 3) ± standard deviation. The sign *, and ** denote significantly differences within the column (p ≤ 0.05).
3.10. In vitro simulated intestinal digestion
In vitro intestinal digestion of noodles incorporated with L. edodes is represented in Table 8. In the present study, it has been observed that in comparison to L1, L3 indicated a significant reduction (p < 0.05) in the release of reduced sugar during the 1 h 20 min digestion. The L3 exhibits an 18 % reduction of reduced sugar release which is greater than the control sample (10 %). However, the L3 exhibits a significantly (p < 0.05) lower reduction of glucose released (40.36 ± 0.75 %) in comparison to the control sample (62.58 ± 0.51 %). This is due to the reason that L. edodes powder limits the amount of carbohydrate digestion in wheat flour noodles. Furthermore, the L. edodes showed a significantly lower (p < 0.05) effect on decreasing the area under the curve with an AUC value of 174.37 ± 1.05 mg/g in comparison to the control (239.68 ± 1.28 mg/g). This is due to the lower concentration of starch content in L3 in comparison to L1. The present results lined with the findings of Wang, Tian, et al., 20211, who studied the effect of glycaemic load manipulation and their correlations with pre-post stimulated in vitro digestion of mushroom noodles fortified with mushroom powder.
Table 8.
The reduction in glucose release (%) and area under the curve (AUC) of reducing sugar release of noodles samples after in vitro digestion.
| Type of sample | Reduction in glucose release (%) | Area under the curve mg reducing sugar/g of noodles |
|---|---|---|
| Control (L1) | 62.58 ± 0.51⁎⁎ | 239.68 ± 1.28⁎⁎ |
| L3 (4 %) | 40.36 ± 0.75⁎ | 174.37 ± 1.05⁎ |
Results are expressed as means (n = 3) ± standard deviation. The sign *, and ** denote significantly differences within the column (p ≤ 0.05).
3.11. Determination of bioactive compounds
The determination of bioactive compounds in both samples L1 and L3 was carried out using spectrophotometric analysis and it has been observed that L3 exhibits significantly (p < 0.05) high concentrations of both phenolic (59.64 ± 0.12 mg/g) and flavonoid content (12.56 ± 0.17 mg/g) in comparison to phenolic components (39.47 ± 0.19 mg/g) and flavonoid components (4.08 ± 0.7 mg/g) of L1as shown in Table 9. The increase in the concentration of phenolic and flavonoid components of L3 is due to the addition of mushroom powder rich in bioactive compounds. The present results are as per the result obtained by Wang et al., 2020 who formulated shiitake mushroom powder incorporated wheat flour noodles and performed the estimation of the total phenolic content.
Table 9.
Determination of total phenolic and flavonoid components.
| Type of sample | Total phenolic components (mg/ g) | Total flavonoid components (mg/ g) |
|---|---|---|
| Control (L1) | 59.64 ± 0.12⁎⁎ | 12.56 ± 0.17⁎⁎ |
| L3 (4 %) | 39.47 ± 0.19⁎ | 4.08 ± 0.7⁎ |
Results are expressed as means (n = 3) ± standard deviation. The sign *, and ** denote differences within the column (p ≤ 0.05).
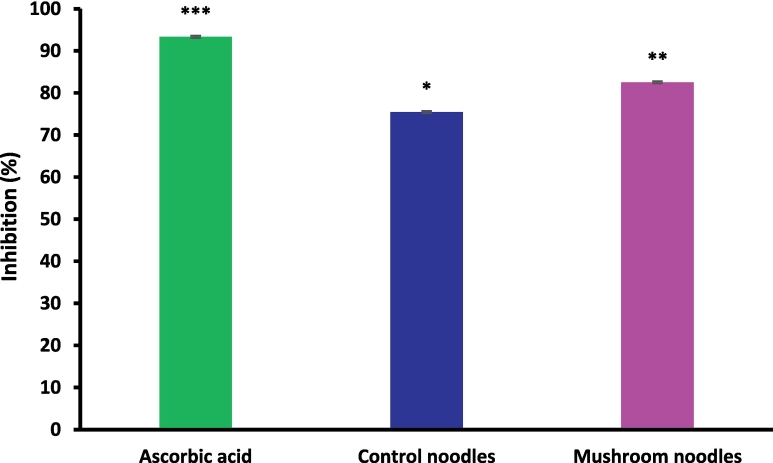
3.12. In vitro antioxidant activity
In vitro antioxidant activity of mushroom-incorporated noodles was evaluated by DPPH free radical scavenging activity and results were given in Fig. 4. In the present study, ascorbic acid in all concentrations detected significantly higher (p < 0.05) inhibition percentages in comparison to control (L1) and noodles incorporated with mushroom powder (L3). Furthermore, L3 demonstrated a high percentage inhibition of DPPH (82.54 ± 0.29 %) in comparison to L1 (75.47 ± 0.27 %). The present results are in accordance with Zakaria et al., 2022, who obtained maximum DPPH free radical scavenging activity for noodles incorporated with 5 % Auricularia cornea mushroom powder.
Fig. 4.
In vitro antioxidant activity of control (L1) noodles and L3 (noodles incorporated with 4 % mushroom powder). The results were expressed as mean ± standard deviation of >3 independent replicates and error bars represent the standard deviation from mean values, while different lowercase letters (*, ** and ***) above each bar represent significantly different (p < 0.05) from each other.
4. Conclusion
The incorporation of L. edodes mushroom powder in noodle formulations has demonstrated a significant (p < 0.05) reduction in the area under the curve (AUC) of reducing sugar release during in vitro digestion. This reduction suggests that noodles containing mushroom powder possess a lower glycemic index, thereby potentially mitigating postprandial blood glucose spikes. Such an attribute is particularly beneficial for individuals managing diabetes or other metabolic disorders, as it contributes to better glycemic control. Additionally, the inclusion of mushroom powder enhances the nutritional profile of the noodles, offering a functional ingredient that aligns with the increasing consumer demand for healthier food products. The findings from this study underscore the dual benefits of nutritional enhancement and improved glycemic response, positioning L. edodes mushroom powder as a viable ingredient in the development of health-promoting noodle products. Future research should aim to validate these in vitro results through long-term clinical trials and investigate the application of mushroom powder across diverse food matrices to fully exploit its functional properties. This study provides a foundation for the broader use of mushroom powder in food systems, highlighting its potential to contribute to public health by offering nutritionally enriched and metabolically favorable food options.
CRediT authorship contribution statement
Subhra De: Writing – original draft. Prince Chawla: Writing – review & editing, Methodology, Formal analysis. Anarase Dattatray: Writing – review & editing, Methodology, Formal analysis. Muzaffar Iqbal: Writing – review & editing, Methodology, Formal analysis. Gulden Goksen: Conceptualization, Validation, Data curation, Methodology, Visualization, Writing – review & editing. Sanju Bala Dhull: Writing – review & editing, Methodology, Formal analysis. Alexandru Vasile Rusu: Validation, Supervision, Investigation, Data curation, Conceptualization. Aarti Bains: Writing – review & editing, Validation, Supervision, Investigation, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors are thankful to the Researchers Supporting Project number (RSPD2024R734), King Saud University, Riyadh, Saudi Arabia for supporting this study.
Support of Central Instrument Facility, Lovely Professional University is gratefully acknowledge.
Contributor Information
Gulden Goksen, Email: guldengoksen@tarsus.edu.tr.
Alexandru Vasile Rusu, Email: rusu_alexandru@hotmail.com.
Aarti Bains, Email: aarti05888@gmail.com.
Data availability
No data was used for the research described in the article.
References
- Ali T.M., Shaikh M., Haider S., Mehfooz T. Development of Gluten-Free Pasta. Academic Press; 2024. Significance of hydrocolloids in the formation of gluten-free pasta; pp. 19–44. [Google Scholar]
- Amaral A.R., Risolia L.W., Rentas M.F., Marchi P.H., Balieiro J.C.D.C., Vendramini T.H.A., Brunetto M.A. Translating human and animal model studies to Dogs’ and Cats’ veterinary care: Beta-glucans application for skin disease, osteoarthritis, and inflammatory bowel disease management. Microorganisms. 2024;12(6):1071. doi: 10.3390/microorganisms12061071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amerikanou C., Tagkouli D., Tsiaka T., Lantzouraki D.Z., Karavoltsos S., Sakellari A., Kaliora A.C. Pleurotus eryngii chips—Chemical characterization and nutritional value of an innovative healthy snack. Foods. 2023;12(2):353. doi: 10.3390/foods12020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC . 17th ed. Association of Official Analytical Chemists; Rockville: 2000. Official methods of analysis. [Google Scholar]
- Arora T., Verma R., Kumar R., Chauhan R., Kumar B., Sharma V. Chemometrics based ATR-FTIR spectroscopy method for rapid and non-destructive discrimination between eyeliner and mascara traces. Microchemical Journal. 2021;164 doi: 10.1016/j.microc.2021.106080. [DOI] [Google Scholar]
- Bains A., Chawla P., Tripathi A., Sadh P.K. A comparative study of antimicrobial and anti-inflammatory efficiency of modified solvent evaporated and vacuum oven dried bioactive components of Pleurotus floridanus. Journal of Food Science and Technology. 2021;58:3328–3337. doi: 10.1007/s13197-020-04891-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltacıoğlu C., Baltacıoğlu H., Seyhan R., Uğur Ö., Avcu O. Investigation of the effect of oyster mushroom (Pleurotus ostreatus) powder on biscuit production and effect on quality criteria by Fourier-transform infrared spectroscopy. Journal of Food Processing and Preservation. 2021;45(2) doi: 10.1111/jfpp.15174. [DOI] [Google Scholar]
- Bhatt S., Gupta M. Formulation of instant noodles incorporated with insoluble dietary fiber from fruit peel: In vitro starch digestibility, biophysical, structural and textural characteristics. Applied Food Research. 2023;3(2) doi: 10.1016/j.afres.2023.100326. [DOI] [Google Scholar]
- Biver E., Herrou J., Larid G., Legrand M.A., Gonnelli S., Annweiler C.…Paccou J. Dietary recommendations in the prevention and treatment of osteoporosis. Joint, Bone, Spine. 2023;90(3) doi: 10.1016/j.jbspin.2022.105521. [DOI] [PubMed] [Google Scholar]
- Chakraborty S., Beura M., Sharma S.K., Singh A., Dahuja A., Krishnan V. Lentinan, β-glucan from shiitake (Lentinula edodes): A review on structure, conformational transition, and gastro-intestinal interaction contributing towards its anti-diabetic potential. Trends in Food Science & Technology. 2023;104224 doi: 10.1016/j.tifs.2023.104224. [DOI] [Google Scholar]
- Chaudhary A., Kumari M., Vyas S. Strategies to combat Iron deficiency Anemia among lactating women in India: A review. Food and Humanity. 2024;100253 doi: 10.1016/j.tifs.2023.104224. [DOI] [Google Scholar]
- Cui C., Wang Y., Ying J., Zhou W., Li D., Wang L.J. Low glycemic index noodle and pasta: Cereal type, ingredient, and processing. Food Chemistry. 2024;431 doi: 10.1016/j.foodchem.2023.137188. [DOI] [PubMed] [Google Scholar]
- Du J., Xi J., Chen X., Sun H., Zhong L., Zhan Q., Zhao L. Effects of different extraction methods on the release of non-volatile flavor components in shiitake mushroom (Lentinus edodes) Journal of Food Composition and Analysis. 2024;128 doi: 10.1016/j.jfca.2024.106001. [DOI] [Google Scholar]
- Haftek M., Abdayem R., Guyonnet-Debersac P. Skin minerals: Key roles of inorganic elements in skin physiological functions. International Journal of Molecular Sciences. 2022;23(11):6267. doi: 10.3390/ijms23116267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Cao Y., Shen Y., Shan Y., Liu J., Song Y., Zhao J. Research progress of edible mushroom polysaccharide-metal trace element complexes. Food Chemistry: X. 2024;101711 doi: 10.1016/j.fochx.2024.101711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman S., Yadav B., Dalal R.C., Naorem A., Sinha N.K., Rao C.S.…Rao A.S. Mushroom farming: A review focusing on soil health, nutritional security and environmental sustainability. Farming System. 2024;2(3) doi: 10.1016/j.farsys.2024.100098. [DOI] [Google Scholar]
- Kała K., Krakowska A., Szewczyk A., Ostachowicz B., Szczurek K., Fijałkowska A., Muszyńska B. Determining the amount of potentially bioavailable phenolic compounds and bioelements in edible mushroom mycelia of Agaricus bisporus, Cantharellus cibarius, and Lentinula edodes. Food Chemistry. 2021;352 doi: 10.1016/j.foodchem.2021.129456. [DOI] [PubMed] [Google Scholar]
- Karimi R., Homayoonfal M., Malekjani N., Kharazmi M.S., Jafari S.M. Interaction between β-glucans and gut microbiota: A comprehensive review. Critical Reviews in Food Science and Nutrition. 2024;64(22):7804–7835. doi: 10.1080/10408398.2023.2192281. [DOI] [PubMed] [Google Scholar]
- Lavanya K., Balagangadharan K., Chandran S.V., Selvamurugan N. Chitosan-coated and thymol-loaded polymeric semi-interpenetrating hydrogels: An effective platform for bioactive molecule delivery and bone regeneration in vivo. Biomaterials Advances. 2023;146 doi: 10.1016/j.bioadv.2023.213305. [DOI] [PubMed] [Google Scholar]
- Li H., Hao Y.P., Dai Y., Chen Z.Z., Ping Y.L., Zhao B.B. Effects of protein-polysaccharide extracted from Auricularia auricula-judae mushroom on the quality characteristics of Chinese wheat noodles. Lwt. 2023;182 doi: 10.1016/j.lwt.2023.114783. [DOI] [Google Scholar]
- Łysakowska P., Sobota A., Wirkijowska A. Medicinal mushrooms: Their bioactive components, nutritional value and application in functional food production—A review. Molecules. 2023;28(14):5393. doi: 10.3390/molecules28145393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng X.Y., Lejaniya A.K.S., Carolyn L.L., Pui L.P. Development of oyster mushroom powder incorporated noodles with wheat, chickpea, and corn flour. Journal of Culinary Science & Technology. 2022:1–22. doi: 10.1080/15428052.2022.2123292. [DOI] [Google Scholar]
- Nordiana A.B., Rosli W.W., Nizam W.W.A. The effect of oyster mushroom (Pleurotus sajor-caju) flour incorporation on the physicochemical quality and sensorial acceptability of pasta. International Food Research Journal. 2019;26(4):1249–1257. [Google Scholar]
- Oyetayo V.O., Ogidi C.O., Bayode S.O., Enikanselu F.F. Evaluation of biological efficiency, nutrient contents and antioxidant activity of Pleurotus pulmonarius enriched with zinc and Iron. Indian Phytopathology. 2021;74:901–910. doi: 10.1007/s42360-021-00410-7. [DOI] [Google Scholar]
- Parvin R., Farzana T., Mohajan S., Rahman H., Rahman S.S. Quality improvement of noodles with mushroom fortified and its comparison with local branded noodles. NFS Journal. 2020;20:37–42. doi: 10.1016/j.nfs.2020.07.002. [DOI] [Google Scholar]
- Patil N.D., Bains A., Kaur S., Yadav R., Goksen G., Ali N.…Chawla P. Effect of dual modifications with ultrasonication and succinylation on Cicer arietinum protein-iron complexes: Characterization, digestibility, in-vitro cellular mineral uptake and preparation of fortified smoothie. Food Research International. 2024;186 doi: 10.1016/j.foodres.2024.114344. [DOI] [PubMed] [Google Scholar]
- Rawat M., Varshney A., Rai M., Chikara A., Pohty A.L., Joshi A.…Gupta A.K. A comprehensive review on nutraceutical potential of underutilized cereals and cereal-based products. Journal of Agriculture and Food Research. 2023;12 doi: 10.1016/j.jafr.2023.100619. [DOI] [Google Scholar]
- Rowaiye A., Wilfred O.I., Onuh O.A., Bur D., Oni S., Nwonu E.J.…Wood T.T. Modulatory effects of mushrooms on the inflammatory signaling pathways and pro-inflammatory mediators. Clinical Complementary Medicine and Pharmacology. 2022;2(4) doi: 10.1016/j.ccmp.2022.100037. [DOI] [Google Scholar]
- Sangeeta S., Sharma D., Ramniwas S., Mugabi R., Uddin J., Nayik G.A. Revolutionizing mushroom processing: Innovative techniques and technologies. Food Chemistry: X. 2024;101774 doi: 10.1016/j.fochx.2024.101774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shams R., Singh J., Dash K.K., Dar A.H., Panesar P.S. Evaluation of cooking characteristics, textural, structural and bioactive properties of button mushroom and chickpea starch enriched noodles. Journal of Food Science and Technology. 2023;60(6):1803–1813. doi: 10.1007/s13197-023-05721-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashikant M., Bains A., Chawla P., Sharma M., Kaushik R., Kandi S., Kuhad R.C. In-vitro antimicrobial and anti-inflammatory activity of modified solvent evaporated ethanolic extract of Calocybe indica: GCMS and HPLC characterization. International Journal of Food Microbiology. 2022;376 doi: 10.1016/j.ijfoodmicro.2022.109741. [DOI] [PubMed] [Google Scholar]
- Singh R.P., Bhardwaj A. β-Glucans: A potential source for maintaining gut microbiota and the immune system. Frontiers in Nutrition. 2023;10:1143682. doi: 10.3389/fnut.2023.1143682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla A., Gupta O.P., Sagwal V., Kumar A., Patwa N., Mohan N.…Singh G. Beta-glucan as a soluble dietary Fiber source: Origins, biosynthesis, extraction, purification, structural characteristics, bioavailability, biofunctional attributes, industrial utilization, and global trade. Nutrients. 2024;16(6):900. doi: 10.3390/nu16060900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sushma A., Kumari K., Narendranath D., Kavitha P. Effect of dietary inclusion of papaya peel powder on serum biochemical and carcass parameters of Japanese quails. Indian Journal of Animal Nutrition. 2023;40(2):218–226. [Google Scholar]
- Vlaicu P.A., Untea A.E., Varzaru I., Saracila M., Oancea A.G. Designing nutrition for health—Incorporating dietary by-products into poultry feeds to create functional foods with insights into health benefits, risks, bioactive compounds, food component functionality and safety regulations. Foods. 2023;12(21):4001. doi: 10.3390/foods12214001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Brennan M.A., Guan W., Liu J., Zhao H., Brennan C.S. Edible mushrooms dietary fibre and antioxidants: Effects on glycaemic load manipulation and their correlations pre-and post-simulated in vitro digestion. Food Chemistry. 2021;351 doi: 10.1016/j.foodchem.2021.129320. [DOI] [PubMed] [Google Scholar]
- Wang L., Tian Y., Chen Z., Chen J. Effects of Hericium erinaceus powder on the digestion, gelatinization of starch, and quality characteristics of Chinese noodles. Cereal Chemistry. 2021;98(3):482–491. doi: 10.1002/cche.10387. [DOI] [Google Scholar]
- Wang L., Zhao H., Brennan M., Guan W., Liu J., Wang M., Brennan C. In vitro gastric digestion antioxidant and cellular radical scavenging activities of wheat-shiitake noodles. Food Chemistry. 2020;330 doi: 10.1016/j.foodchem.2020.127214. [DOI] [PubMed] [Google Scholar]
- Zakaria M.K., Matanjun P., George R., Pindi W., Mamat H., Surugau N., Seelan J.S.S. Nutrient composition, antioxidant activities and Glycaemic response of instant noodles with Wood ear mushroom (Auricularia cornea) powder. Applied Sciences. 2022;12(24):12671. doi: 10.3390/app122412671. [DOI] [Google Scholar]
- Zhang Z., Zhao L., Qu H., Zhou H., Yang H., Chen H. Physicochemical characterization, adsorption function and prebiotic effect of chitin-glucan complex from mushroom Coprinus comatus. International Journal of Biological Macromolecules. 2022;206:255–263. doi: 10.1016/j.ijbiomac.2022.02.152. [DOI] [PubMed] [Google Scholar]
- Zhong S., Kopec R.E. Bioaccessibility and Caco-2 cell uptake of iron chlorophyllin using a biologically relevant digestion model. The Journal of Nutritional Biochemistry. 2024;132 doi: 10.1016/j.jnutbio.2024.109698. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.