Abstract
Patient involvement is crucial in healthcare, a factor increasingly recognised by life sciences companies and research institutes. This article presents a case study on Servier, a life sciences company that founded a patient expert board, ahead of launching a new research and development (R&D) institute. The aim was to foster a patient-centric culture within the company. The case study explores key developments in patient and public involvement, emphasising a shift from paternalistic to patient-centred approaches, noting few available case studies on patient board collaborations in life sciences. It outlines the evolution of the board, its impact, and practical lessons learned, with related recommendations. The patient board resulted from a three-way collaboration between the company, Patvocates (a patient consultancy), and patient experts recruited. The patient consultancy played a crucial role in project management, governance, and facilitating relationships. The case study provides the context, timeframe, foundations laid, engagement of patient experts, and foundational values, including: co-creation, fair market value remuneration, voluntary participation, and patient-centric meeting protocol. Eighteen patient experts, representing ten disease areas and ten European countries, joined the board and helped prioritise and co-create projects. Ideas for activities were sourced from brainstorming sessions and an in-company challenge. The collaboration yielded five core ideas, each forming a working group. The study describes the groups and their outputs: a patient advisory council, an interactive gallery of patient experience in R&D, patient engagement and entrepreneurship in life sciences, creating patient-focused decentralised trials (DCTs), and staff training on patient engagement. The article emphasises how the organic evolution of the collaboration led to significant insights. Hurdles faced by the company included: upstream planning, cross-company buy-in, compliance, and internal resource allocation. Recommendations for the wider community included: identifying and contracting patient partners; clarifying roles; managing expectations; building trust; logistics; and sustainability. This case study presents a practical, positive example of patient engagement within a life sciences company, offering insights into the establishing, running, and the impact of collaborating with a patient expert board. Lessons learned and recommendations may serve as a model for other companies seeking to engage with patients and evolve towards a more patient-centric approach in their strategies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40900-024-00631-w.
Patient involvement in healthcare is crucial for developing patient-centred approaches, and life sciences companies and research institutes are increasingly recognising this. Servier, a life sciences company, established a patient expert board to support a patient-centric culture within the organisation, at the time of creating a new research and development (R&D) institute. This article presents a case study on the patient board and its impact. The patient board resulted from a three-way collaboration between the company, Patvocates (a patient consultancy), and patient experts recruited. The patient consultancy played a key role in guiding the project. The study provides the context, founding values, engagement of patient experts, and methodology used to establish the board. Eighteen patient experts, representing ten disease areas and ten European countries, joined and helped prioritise and co-create projects. From the collaboration, five core ideas emerged. The case study highlights that the organic evolution of the collaboration provided significant insights. Hurdles faced included cross-company buy-in, compliance, time, and resources. The study also offers a set of recommendations for the wider community, including identifying and contracting patient partners, clarifying roles, managing expectations, building trust, logistics, and sustainability. This case study presents a positive, constructive model of patient engagement within a life sciences company, offering insights into establishing and running a patient expert board and its impact on the company culture and R&D practices. The lessons learned and recommendations may serve as a model for other companies wanting to engage with patients and develop more patient-centric approach in their strategies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40900-024-00631-w.
Introduction
Patient and public engagement evolving from the mid-1900s onwards
Patient and public engagement in global healthcare and public health has evolved progressively from a historically passive role towards a participative and active model [1]. This development has several driving forces and is increasingly covering different aspects of health, including R&D. The foundations for this evolution were laid from the mid-1900s onwards.
In 1946, the World Health Organization (WHO) Constitution redefined health and the importance of patient and public engagement. When the WHO Constitution was adopted [2], a new definition of health was established: ‘Health is a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity.’ This definition has paved the way to a more complex, deeper, and patient-centric understanding.
Simultaneously, the WHO Constitution emphasised the value of the active education and engagement of people, the public, in their own health. Together, these two points highlight an increasingly holistic and multidisciplinary view of health and the growing involvement of patients in their own health management, which has since become more widespread practice.
Regulatory bodies catalysing patient involvement in medicines R&D
The journey towards patient-centricity has gained momentum due to shifts in the regulatory landscape, with regulatory agencies increasingly seeking input from patients to inform decision-making [3]. The U.S. Food and Drug Administration (FDA) has actively promoted patient engagement through several initiatives. These include Patient-Reported Outcomes (PROs) from 2009 onwards; a discussion document on patient engagement in medical device clinical trials in 2018 [4], with a resulting guidance published in 2022 [5]; and a pioneering four-part guidance series on patient-focused drug development [6], the first of which, in 2018, focused on collecting comprehensive and representative input, including from patients [7].
In a guidance aimed at the pharmaceutical industry published in 2023, the FDA stated that: patients are the ultimate stakeholder in the outcomes of medical treatments; that it is developing systematic approaches to better incorporate the patient voice in medicines development and evaluation; and that patient experience data (PXD) collected early can help identify unmet patient needs [8].
Similar trends in Europe include the EU Clinical Trials Regulation 536/2014 and patient involvement in decision-making at the European Medicines Agency (EMA) [9]. After at least a decade of groundwork, the EMA launched its Public Engagement Department in 2014, emphasised the involvement of young patients in 2017 [10], and in 2022 published a revised version of its guiding framework, Engagement Framework: EMA and patients, consumers and their organisations [11].
A 2024 international review of patient engagement and PXD across different stakeholders in different regions, including regulatory and Health Technology Assessment agencies, showed encouraging developments in guidance and policies. Input from more than 50 initiatives indicated, however, that further operational and standardised processes were still needed to ensure global integration across different contexts [12].
A shift away from a traditional, paternalistic model of healthcare
The pre-existing, paternalistic healthcare system was largely focused on the ‘consultation’ (medical appointment), a transaction between a patient and a healthcare provider, in which the latter assumed a dominant role throughout the interaction, including on decision-making [1]. This interaction, while typically taking place individual-to-individual, represented the interface between the healthcare system and broader society.
At a higher level, traditional healthcare systems have also determined the health outcomes of patients in a similarly paternalistic way, offering individuals little opportunity for engagement. This traditional approach has largely ignored patients’ opinions and needs, excluding them from decision-making and other types of exchanges [1].
New models of collaboration between health professionals and patients have led to the concept of the ‘patient-centred’ and ‘patient-centric’ healthcare system [13]. One of the main differences of the model is the introduction of shared decision-making, which has proved effective in the implementation of health programmes. Patient organisations have also played a key role, driving positive change towards a shared and collaborative decision-making model [14].
Furthermore, the importance of the patient as an individual resonates in other aspects of healthcare. For example, medicine has moved from empirical approaches to personalised medicine, and from clinical trials focused on capturing clinical endpoints to including PROs. These changes fuel an ever-growing need for patient engagement in R&D.
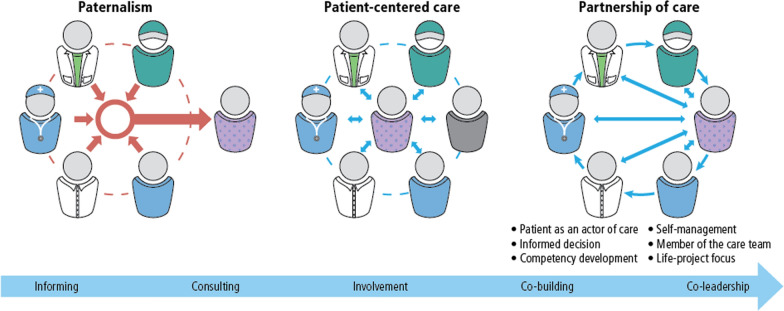
Table 1 defines relevant terms and concepts used in this article, providing relevant published definitions. Figure 1 illustrates a considerable evolution in approaches: from the paternalistic (healthcare providers and health systems toward the patient, offering information and recommendations), to a more patient-centred approach (increasingly enabling patients, with their family and caregivers to make informed decisions). The third stage of development illustrated is partnerships of care, in which interactions are increasingly guided by patient preferences, reciprocal exchanges and dialogue, and ideally by collaborative, shared decision-making.
Table 1.
Table of definitions
| Term | Definition and source |
|---|---|
| Caregiver (also caretaker) | A person who helps a patient with daily activities, healthcare, or other activities that the patient is unable to perform because of age, illness or disability, and who understands the patient’s health-related needs. This person may or may not be a family member and may or may not be paid [6]. |
| Co-creation | Co-creation refers to the collaborative approach of creative problem solving between diverse stakeholders at all stages of an initiative, from the problem identification and solution generation through to implementation and evaluation [16]. |
| Life sciences industry | The life sciences industry comprises companies operating in the research, development and manufacturing of pharmaceuticals, biotechnology-based food and medicines, medical devices, biomedical technologies, nutraceuticals, cosmeceuticals, food processing, and other products that improve the lives of organisms [17]. |
| Medicine life cycle | The time between the first discovery of a potential medicine to when the medicine, once developed, is no longer available to patients [9]. |
| Patient | Any individual with or at risk of a specific health condition, whether or not they currently receive any therapy to prevent or treat that condition. Patients are the individuals who directly experience the benefits and harms associated with medical products [6]. |
|
Patient-centricity (also patient-centredness) |
Putting the patient first in an open and sustained engagement of the patient to respectfully and compassionately achieve the best experience and outcome for that person and their family [18]. |
| Patient community | [This] broadly encompasses individual patients, family caregivers, and the organisations that represent them. The patient community is heterogeneous and brings to the discussion different perspectives informed by their experiences, trajectory or stage of disease, level of expertise, and many other personal, community, and societal factors [9]. |
|
Patient engagement (also patient involvement) |
The active, non-tokenistic and collaborative interaction between patients, the patient community and other stakeholders, where decision making is guided by patients’ contributions as partners, recognising their unique experiences, values and expertise [9]. |
| Patient expert | A person living with a health condition whose knowledge and experience enables the person to take more control over personal health by understanding and managing the health condition. Expert patients may also act as advocates for their condition and help other patients with the same health issue [9]. |
| Patient organisation | An institution that represents the interests and needs of patients (and their families and caregivers) who have a particular disease, disability or group of diseases and disabilities. Patient organisations may engage in research, education, advocacy, and fundraising to further the needs of their patient group [9]. |
| Patient partner | An individual patient, caregiver, or patient group that engages other stakeholders in various capacities to ensure the patients’ wants, needs, and preferences are represented in activities related to healthcare decision making, policy, research and development, and treatment access [19]. |
| Patient-reported outcome | Data reported directly by the patient about aspects of their health without prior interpretation of the patient’s response by a clinician or anyone else [9]. |
| Patient voice (also patient insights) | The input and perspective of patients on their needs and what is of value to them, which can differ from needs identified by other stakeholders (e.g. medicine developers, physicians, regulators, and payers) [9]. |
Fig. 1.
The evolution of patient care, from a paternalistic to patient-centric and partnership models
Source Pomey and Lebel, 2016 [15]
Patients as drivers in healthcare, identifying new needs and broadening scope
From around 2000 onwards, the concept of P4 medicine emerged: predictive, preventive, personalised, and participatory [20, 21]. P4 medicine aimed to move beyond a one-size-fits-all approach to medicine to a more individualised and proactive model, considering the unique genetic, environmental, and lifestyle factors influencing an individual's health. Notably, ‘participatory’ indicates the role of the individual in optimising their health, too.
Patients are increasingly perceived, not only as the ultimate beneficiary or end-user of health technologies, but as a key driver within healthcare systems, of improving value, and of identifying unmet needs. New unmet needs, which can be identified together with patients, can provide new insights, innovation, and momentum for making patient engagement more common practice [22].
Patient-centricity is gradually reaching the domain of medical training, in certain settings. Patient-centred care has evolved into a substantial component of undergraduate medical programmes preparing professionals for applying it in clinical practice. Indeed, systematic reviews show that patient-centred care results in increased adherence to management protocols, reduced morbidity, and improved quality of life [23].
The shift towards patient-centricity thus provides a broader scope, going beyond defining health priorities for patients and the care services they may access. In the past decade, it has spread to several other health-related areas, including medical research—classically contained within academia, the life sciences industry, and regulatory bodies [24].
The evolving concept of ‘the patient’
Similarly, the concept of ‘the patient’ has changed from a person who is living with a disease to ‘patient expert’ with high-level expertise of its daily ramifications. This expands to a ‘patient partner’ which can include caregivers, members of the public, or patient groups, representing the patient’s needs across diverse scenarios (Table 1).
The concept of the patient has thus evolved from a generic or individual patient to a more collective or generalised representation, emphasising the broader significance of patient perspectives, and involvement, including as an actor advocating for changes in healthcare practices and policies. It may also include patient ambassadors, public figures, or key opinion leaders who can play a role in reducing disease-related stigma, empowering patients, and driving positive change such as ‘pro-patient policies’ [25]. When considering needs and preferences and expertise of patients, we increasingly consider the whole support system, including family and caregivers.
Partnering with patients of varying and increasing expertise and skills
As the definition in Table 1 indicates, the skills of patient partners may vary considerably. Figure 2 illustrates how, as such, they may be grouped within four ‘types’, depending on their: personal (disease) experience; understanding of technical matters; professional technical expertise gained in patient engagement; and connection with the broader patient community [26].
Fig. 2.
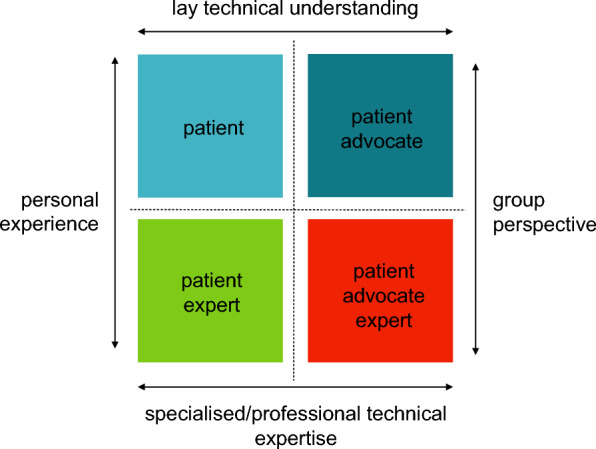
Types of partners and expertise: patients, patient experts, patient advocates, and patient advocate experts.
Source Bettina Ryll, Melanoma Patient Network Europe, 2022 [26]. (Reproduced with permission under Creative Commons License CC BY-NC-SA 4.0)
To ensure that patient collaborations are meaningful, appropriate and useful, it is important to identify the skills and knowledge required for participation and to fit the right person to the right task.
The emerging prerequisite that research be patient-centric to obtain funding
Medicines R&D, whether conducted through the pharmaceutical industry or in other settings, is strongly driven by trends, practices, and discoveries from academia, in universities and their hospitals, and from other research institutions. While in certain settings and countries, academic and research institutions have adopted patient-centricity, in others the practice is still emerging. Funding bodies are increasingly requiring that R&D demonstrate that it incorporate patient and public involvement, and that the results of funded research be disseminated to the public in a patient-centric manner [27].
The impacts of patient engagement and of related measurable outcomes in the medicine lifecycle
The potential impacts of patient engagement have been written about in numerous publications. Positive impacts include: improving discovery, development, and evaluation of new effective medicines by identifying and understanding better the unmet needs of patients; establishing research priorities based on unmet requirements; optimising clinical trial design, outcome measures, and endpoint development; and improving the recruitment and retention of participants in clinical trials [3, 28, 29].
The impact of patient engagement on clinical research and its performance has been increasingly quantified, using different metrics, and showcased. For instance, the incorporation of patient insights in clinical trial design has the potential to prevent protocol amendments and enhance enrolment, patient adherence, and retention. These factors may significantly reduce the development cost of new drugs and reduce times to product market launch [29].
Going forward, further development of patient-centric measures and their systematic implementation across the R&D process will be needed to open up new perspectives on traditional models of R&D, in particular the value of incorporating patient insights [3].
A lack of published case studies on patient engagement in the life sciences industry
Despite this shift in mindset becoming more widely accepted, its practical application remains uncharted territory for many stakeholders across the healthcare spectrum [30]. When systematic reviews were performed, although the feasibility of the patient engagement process was confirmed, a certain lack of consistency with regard to final outcomes and standardisation of approaches was observed [18, 25, 31]. Indeed, some organisations have even pointed to a high level of confusion around how to operationalise a patient-centric approach or achieve the necessary culture change (including matters such as legal issues and conflict of interests) [29].
The life sciences industry thus faces this challenge: How can patient-centricity be embedded in their work and company culture? Patient-centricity represents an emerging mindset, unfolding and developing in situ, being co-created, as it is applied. Yet there are relatively few peer-reviewed published cases to showcase how collaboration can take place between life sciences’ companies and patients, to provide inspiration, models, or lessons learned for the public, and for other companies [32]. Reputable, co-created frameworks for monitoring and evaluation exist and they need to be applied to case studies, and related findings and learnings shared [33–35]. The case study below provides an example of such a collaboration and an opportunity to learn from its recommendations.
Methodology
Introducing the case study on the Servier patient board
It is against this background, and in pursuit of fostering patient-embedded research and driving a cultural shift within the organisation, that Servier (hereafter, ‘the company’) embarked on a transformation of its company culture in 2019, aiming to transition towards a patient-centric model [36].
The company has stated publicly its aims to integrate the patient’s voice at the heart of its activities, from research to support ‘beyond the pill’ [36]. To this end, it created different structures devoted to patient advocacy and engagement, and adapted existing processes to facilitate collaborations with the patient community.
This article serves as a case study, to describe how a patient board was founded, the board governance processes, the activities, its progress and achievements, and its impact on the company culture: the company’s approach to medicines R&D and to the conceptualisation of the institute. For this type of article, we cannot follow traditional, scientific research methodology. Rather, we aim to provide readers with both a practical case study and an opportunity to explore lessons learned and recommendations of potential use for other organisations who would like to integrate patient engagement in their internal strategies. We believe that the level of detail provided in this article could be useful for readers interested in implementing similar projects.
The context: planning to launch an R&D institute
Servier was planning to launch towards the end of 2023 a new R&D premises, the Paris Saclay R&D Institute (hereafter ‘the institute’). This institute is located in Saclay, south-west of central Paris.
Instead of creating a classical R&D infrastructure, typically providing access to qualified employees only, the company envisioned a cross-disciplinary hub. They aimed for it to be accessible to the public, linked to the local scientific community, including a biotechnological incubator, and guided by a clear patient-centric vision.
Creating a patient expert board within the company
The company thus took this opportunity to accelerate its patient-centricity transformation programme, involving different stakeholders from the patient community. This transformation included the creation of the Servier Saclay R&D Patient Expert Board (hereafter ‘the patient board’ or ‘the board’), considered to be an ideas-generation phase, to guide the process and to see it to realisation.
By creating the patient board, the company was striving to embed a new collaborative model. It required a collective change, from a traditional to a forward-looking way of working, in which patients are recognised as a key stakeholder and driver [37, 38].
Timeframe of the case study: 2021–2023
The board was initiated in June 2021, close to two years before the opening of the institute in February 2023. The company’s goals in establishing the board included to gather insights, identify priorities, and catalyse different perspectives with which to forge a strategic plan.
The board concluded the process of ideas-generation and implementation of first proposals two years later, towards the end of 2023, when the new R&D institute was formally opened. Of the 18 patient board members initially engaged, 12 visited the institute in person, to review and reflect on the progress made by the board.
Initial steps of the company’s ‘Patient-In Action Plan’
Upstream, from 2019 on, the company set out a programme to assess the staff’s exposure and knowledge regarding patient engagement activities. This programme, called the ‘Patient-In Action Plan’, took place internally over three stages: training, a survey, and a challenge.
After two training modules sourced from Patient Focused Medicines Development (PFMD) were held, the company held an internal survey, asking 400 R&D staff about their previous experience in patient engagement. Results indicated that despite interest in the topic, only one-third (35.2%) of respondents had had some previous experience. The need to adapt internal processes and resources was thus identified.
Worldwide staff, from diverse departments in the company, were then invited to an internal challenge, calling for projects and suggestions of activities to carry out in the new research institute, under the topic of patient engagement in R&D. A total of 84 ideas from different teams were proposed for consideration.
The role of the patient consultancy in a three-way collaboration
The board was founded on a three-way collaboration: the company, a patient consultancy, and the patient board. To set it up, the company worked closely with Patvocates, a patient consultancy, think-tank, and social enterprise founded and run by patient advocates (hereafter ‘the consultancy’) [39]. The consultancy facilitates relationships between patients and patient organisations with third parties seeking to collaborate with them. Crucially, in its own governance model, the consultancy embodies leadership by, with, and for patients, which helped generate trust from the patient board.
The consultancy was involved from the initial stages of the project management, governance, and strategy. Beyond facilitating engagement with patients and the processes of the board, it provided expert patient advice, including insights gained from lived experience as patient advocates. Indeed, half of the members of the consultancy involved had prior experience as patient advocates.
A core team managing the overall process was made up of two company employees and three members of the consultancy. The two company employees attended all board meetings and activities. Additionally, ten other company staff attended ad-hoc meetings as occasional observers, to foster company-wide learning about the process of patient engagement.
Engaging patient partners via different channels
To identify experienced and skilled patient experts, the company approached the European Patients’ Academy on Therapeutic Innovation (EUPATI). EUPATI, a public–private partnership founded in 2012, aimed to become a ‘game-changer’ for patient empowerment in Europe and beyond [30, 40]. EUPATI focuses on education and training to increase the capacity and capability of patients to understand and contribute to medicines R&D.
Notably, as of early 2024, EUPATI has trained up a body of 331 patient experts who have graduated as EUPATI Fellows [41, 42]. The company issued a proposal via EUPATI’s matchmaking tool, EUPATI Connect (formerly the EUPATI Matchmaking Platform). It was thus from this pool of Fellows that most of the board members were recruited. Further patient experts were recruited via the consultancy and the company’s network. Due to the nature of this project, there were no formal inclusion or exclusion criteria in the recruitment process. All participants, however, received information about the level of involvement and technical abilities required, the objectives, and the possibility to leave, if desired.
Make-up of the patient board: numbers, countries represented, and disease areas
Initially, 18 board members were recruited. Two patient experts left the board in the second year due to personal reasons. A total of 16 (88.8%) remained engaged through to the end of the ideas-generation phase of board activities.
The 18 patient experts recruited represented ten countries of residence across Europe: Austria, Belgium, France, Germany, Ireland, Italy, Portugal, Spain, Switzerland, and the United Kingdom.
The ten disease areas represented were:
Attention deficit hyperactivity disorder (ADHD)
-
Cancer
- Breast cancer
- Head and neck cancer
- Hereditary cancers
- Melanoma
- Non-Hodgkin lymphoma
- Ovarian small cell carcinoma
- Paediatric cancers
-
3.
Cystic fibrosis
-
4.
Endometriosis
-
5.
HIV
-
6.
Paediatric illnesses
-
7.
Parkinson’s disease, including young onset
-
8.
Relapsing–remitting multiple sclerosis
-
9.
Sjögren disease
-
10.
Traumatic brain injury
Foundational values of patient engagement
To establish the patient board, the key founding values, based on the PFMD Quality Criteria for Patient Engagement [43, 44] were agreed and used by the group. The PFMD guidance proposes seven foundational criteria (values), appearing in Fig. 3: 1. shared purpose; 2. respect and accessibility; 3. representativeness of stakeholders; 4. roles and responsibilities; 5. capacity and capability of engagement; 6. transparency in communication and documentation; and 7. continuity and sustainability.
Fig. 3.
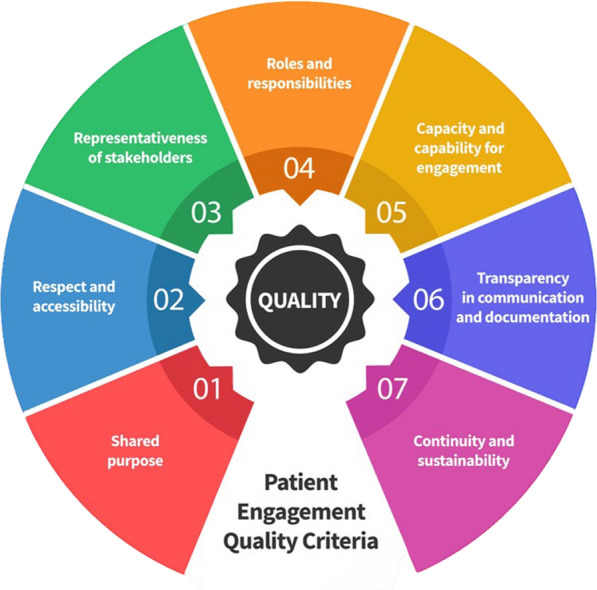
Seven patient engagement quality criteria.
Source PFMD, 2018 [43]. (Reproduced with permission under Creative Commons License CC BY-NC-SA 4.0)
In addition to these founding values, further principles integrated were: co-creation; fair remuneration according to local compliance rules; and voluntary opt-in and opt-out participation on the different projects proposed.
Exploring the value of co-creation
Co-creation is a core principle of patient engagement. It can be described as ‘the collaborative approach of creative problem solving between diverse stakeholders at all stages of an initiative, from the problem identification and solution generation through to implementation and evaluation’ [16]. Integral to co-creation is a two-way exchange and feedback loop, promoting learning while doing and adjustment throughout the process.
Quotes from group participants, reflecting on their experience of this co-creation process and the integration of the patient voice, are provided in Table 2. While these quotes were a spontaneous expression, we recognise that there is scope for a more systematic qualitative assessment of participant experience, cross-checking with the foundational values.
Table 2.
Reflections on the working group collaborations from board and consultancy
|
1 Patient Advisory Council In the Patient Advisory Council, we advocated for patients to be genuine partners in all R&D stages, beginning early on. Our goal was to ensure their involvement is structured, suitable, and valuable, adhering to best practices and fairness in remuneration. This approach seeks mutual benefit, improving both patient outcomes and the R&D process, while authentically representing the needs of those with specific conditions. Collaborating with the Servier team has been a positive experience, observing their commitment to fostering meaningful changes through an open and motivated mindset shift and action. Patient expert 1 |
|
2 An Interactive Gallery of Patient Experience in R&D In this working group, we wanted researchers – working with molecules, over microscopes in labs, really far upstream from patients – to hear some of our stories. So we came up with the idea of patient testimonials that could be told by video, podcast, or art, to help bridge this divide. We want researchers to feel that their work will reach real people one day. We want them to know our hopes that innovation through R&D could improve our lives. Patient expert 2 |
|
3 Patient Engagement and Entrepreneurship in Life Sciences Working closely with Servier on the start-up incubator, we patient board members set the shared goal of incorporating patient insights where they are often missing – in the biotechnology start-up setting, from early to late stages. Patients can bring enriching ideas, providing further areas for entrepreneurs to explore. It was challenging, but our working group fostered opportunities to transform ideas, aiming to make the entrepreneurial projects more patient-centric. We hope to help these projects be both innovative and sustainable. Patient expert 3 |
|
4 Patient-Focused DCTs Being involved as a partner with the R&D professionals from Servier in this working group has allowed interactive discussions about the options and opportunities that the decentralisation and digitalisation of clinical trials can offer to benefit patients. During the conception of each individual study is where this dialogue with expert patients and patient representatives can be an asset to ensure the right decisions are made, in terms of providing the best experience for patients and ensuring their adherence while participating in a clinical study. Patient expert 4 |
|
5 Training on PE As for the patient engagement training group, we were keen to have some basic ‘What is patient engagement?’ training taking place throughout the company. This way, all the staff could get a taste of what this buzzword means. The next step was to hold customised training for staff involved in R&D, where patient insights could affect their work. It was really encouraging to see how interested and open these staff were. Patient expert 2 |
|
Consultancy perspective To ensure co-creation, shared purpose, and patient impact were at the core of engaging the patient community in the Servier Saclay R&D Patient Expert Board, Servier collaborated with our patient-led thinktank and expert consultancy on patient engagement, Patvocates. Our Patvocates team supported the company in establishing and facilitating the board and working groups, strengthened capacity and capability for engagement on both sides, and made sure that the engagement was based on key values like honest and transparent communication, equal-to-equal partnership, and thorough follow-up and continuity. It was amazing to see the results, the intensity, and tangible outcomes of the interactions. Patient consultancy member |
Board members and the member of the consultancy granted their permission for these quotes to be published
Drawing on fair market value to remunerate patient experts
Remunerating patient experts, especially when they live in different countries under different regulations, requires careful consideration and planning. Over many years, it has become an increasingly common practice and several guidelines have been developed [9, 45].
To align with fair and transparent remuneration, regarding participation in the patient board, the company considered local compliance regulations and fair market value. The countries of residence of patient board members or their patient associations were taken into consideration.
Fostering voluntary opt-in and opt-out participation
All activities were proposed to the patient board. Patient experts were then free to choose working groups and projects that interested them, regardless of their expertise, geographical location, or disease affiliation. Patient board members were also free to leave the project at any time and with no obligation to provide a reason.
Refining meeting protocol and enabling input into summary reports
Due to the disruption of the Covid-19 pandemic, patient board meetings were held purely via videoconference. This meant patient board members could attend, irrespective of their mobility and ability to travel.
Early meetings were facilitated by using digital brainstorming tools, which were simplified as the project advanced, to encourage the participation of all group members. Goals included: identifying key priorities; arranging them into suitable thematic working groups and identifying relevant aims; and matching participation to individuals’ skills and interests.
Ahead of each meeting, board members received an agenda and pre-reading. During the meetings, participants could add comments, suggestions, and relevant links in the chat functions. This was important for patient partners who had difficulties speaking or with the working language of the meetings, English. Meetings were recorded for note-taking or for replay. Board members received meeting summaries afterwards, for their comment, or amendments, and reports were amended, according to feedback.
Results
In this section, we define results as what the co-creation process yielded, both in ideas and in outputs: from defining five broad areas to five specific working groups, plus a plenary group. We provide a summary of the goals, the activities and progress resulting from each group, and a snapshot of its members.
Selection of projects and priorities
Results of the board activities, the main projects, and priorities of the engagement were identified and selected via two sources:
Brainstorming sessions organised between the board, the company, and a facilitator: over two sessions, key ideas from board members were identified and voted on.
Ideas from the company’s internal challenge: as mentioned above, 84 ideas from within the company were shared with the patient board and then voted on. Decisive criteria for voting were: interest for the patient community; timing; feasibility; input on research; and input on the broader patient community.
Five core ideas identified and related working groups formed
To ensure a progressive implementation of proposals, five core ideas were retained from the topics most voted on. Five working groups were then established, which included members of the patient board, company staff and, as needed, facilitators from the consultancy. Over the collaboration, 18 meetings were held.
Brief descriptions of the five resulting working groups (goals, activities and progress, and number of participants per group) appear below. For all working groups, however, activities have since extended beyond those described.
Working group 1. patient advisory council
Goal
The patient board expressed an interest in creating, by disease area, a consultative patient group to accompany project development of new therapies by the company. The group aimed to situate, systematise, and structure the patient voice in the company’s therapeutic projects by creating standardised processes to support R&D teams, from early stages and along the development plan, until post-commercialisation of the given therapy.
Activities and progress
The group accomplished the objective of creating a detailed process: from identifying patient communities, to topics to be discussed, type of members to include, and both rules of exchanges for the organisation and timing of implementation of key steps in the research programme.
Forthcoming: At the time of writing, the company had started to pilot this process, which remained flexible, and aimed to apply it to three indications or disease areas.
Working group members:
8 patient board members and 4 company employees.
Working group 2. an interactive gallery of patient experience in R&D
Goal
The patient board expressed an interest in creating a visual representation of patient-centricity inside the institute. Approaches suggested including exploring the use of shared spaces and translating different elements of the common vision, about the impact of medical research on patients’ lives, into interactive, multichannel media.
Activities and progress
An art exhibition, showcasing art produced by patients, was held on-site for institute staff and visitors.
A co-created podcast series, ‘The Patient’s Side of the Story’, showcasing patient stories and exploring how R&D and innovation can benefit patients, was launched and shared with company and institute staff, patients, their families/caregivers, and the public.
A series of conferences, cinema, and a theatre play about living with chronic illness were held periodically in the Saclay neighbourhood and attended by company and institute staff.
Forthcoming: A series of art works to express patient feelings about their disease is being co-produced between patients and consolidated artists, displayed on-site, and featured in the podcast series.
Working group members:
11 patient board members and 3 company employees.
Working group 3. patient engagement and entrepreneurship in life sciences
Goal
This project aims to develop patient engagement for biotechnology and to support patient entrepreneurs. The patient board expressed an interest in co-creating and developing processes and rules for involving patients in Spartners, an incubator operated by BioLabs and Servier, that serves as a membership-based network of facilities to support resident start-ups [46]. The incubator aims to introduce these resident start-ups to the fundamentals of patient engagement and the value of integrating patient views in their projects.
Approaches suggested from early- to late-stage involvement included: bringing insights from patients’ lived experience and expertise; exploring synergies across disease areas; participating in reflection, discussion, and design; and learning about practical challenges in this domain. In addition, through the above-mentioned internal challenge, employees expressed the wish to define models and rules that could enable them to support patient entrepreneurs, while adhering to rules of compliance.
Activities and progress
The working group developed a guidance and a patient engagement training plan for entrepreneurs and start-ups incubated in Spartners, thereby fostering patient-centric dialogue at the earliest stage of start-up activity. This enables them to benefit from Servier’s learnings in patient engagement.
The working group ran brainstorming sessions to identify ways in which initiatives led by patients (or their relatives) could be identified, evaluated, selected, and supported; it is currently defining the format by which patient initiatives can be supported.
Forthcoming: Further projects to foster patient-driven entrepreneurship are being developed.
Working group members:
7 patient board members and 8 company employees.
Working group 4. patient-focused decentralised trials (DCTs)
Goal
The patient board expressed an interest in co-creating guidelines on patient-centric running of decentralised clinical trials (DCTs), as an option for certain forthcoming trials sponsored by the company.
Activities and progress
The working group carried out brainstorming to explore the opportunities, strengths and weaknesses, and concerns of patients related to DCTs, notably those defined as hybrid (combining traditional and decentralised approaches to trials).
The working group proposed and consolidated six principles relating to the process of co-designing hybridised DCTs. It co-created a guidance document, which was then applied to a pilot project, relating to a rare cancer study.
Working group members:
9 patient board members and 4 company employees.
Working group 5. training on patient engagement
Goal
The patient board expressed an interest in having company staff trained on soft and hard skills needed to facilitate incorporating the principles of patient-centricity into the company’s R&D projects. This training was linked to a broader goal of changing the mindset within the company, relating to patient engagement.
Activities and progress
The working group consolidated a patient engagement training programme to be used by company R&D collaborators.
A training course on the fundamentals of patient engagement was developed and delivered to R&D professionals, together with patient experts from EUPATI.
Forthcoming: Training on soft skills is in development.
Working group members:
7 patient board members and 3 company employees.
Plenary activities: Servier Saclay R&D Patient Expert Board
Goal
As a plenary, the patient board met to advance the patient engagement ideas, to vote for projects, and to receive updates on plans, including on the five working groups.
Activities and progress
The patient board members took part in working groups of their individual preference, and some expressed interest in participating in a resulting publication. The result was this article, patient-led, involving co-authorship with employees from the company and consultancy, and patient contributors.
Most of the patient board visited the institute in person, a visit which marked the end of the ideas-generation phase. Thereafter, the company offered to the board the opportunity to collaborate on the R&D patient engagement strategic plan for 2024 onwards, which is now being implemented. Most of the board members expressed a wish to continue providing ideas for future collaborations.
Working group members:
First 18, then 16 patient board members, 2 company employees, and 3 consultancy staff.
Integration of the patient voice into working group development and outputs
Throughout the collaboration, patient board members were consulted, and their opinions noted. Table 2 provides quotes from the patient board and the consultancy, indicating their reflections on the collaboration.
Discussion
The period of collaboration enabled all members of the company, the consultancy, and the patient board to experience a work in progress–its opportunities, challenges, and the ways in which difficulties were navigated and negotiated.
Because this process was relatively organic, it led to considerable learning on challenges and an opportunity to try out solutions and identify recommendations for best practices. These learnings are discussed below.
Challenges unique to the company, as part of the life sciences industry
Life sciences companies who are interested in creating patient boards face challenges unique to their industries. These include:
Upstream planning: Laying the foundations within the company with a good lead time will facilitate its internal buy-in. This requires integrating patient-centricity in its strategic vision and commitment at a senior-management level, exploring how it will relate to R&D goals of the company, and developing an initial plan. Servier communicated this to the public and its stakeholders via its Annual Reports, with increasing level of detail, from 2020, before the board was created. While this process is not the focus of this case study, it could be of interest for future publications to share good practice and learnings [36, 47].
Uneven cross-company buy-in: By ascertaining internal levels of awareness and interest, which were varied (as can be expected in large organisations), the company was able to lay the foundations for patient engagement. These foundations enabled that which followed to be meaningful, impactful, and authentic. Conducting any activity for the first time is challenging and calls for motivation and commitment. Having the endorsement of senior management facilitated buy-in from other staff members.
Compliance: Because the patient board members were living in several countries, different compliance regulations applied to how the company was permitted to interact with them. It was thus challenging to find a balance between defining meeting agendas upfront and enabling dynamic brainstorming sessions. This required the company to provide transparent feedback to board members on what suggestions were likely to be integrated into the given working group’s projects. Having clear, standardised internal procedures for engaging with patient experts, locally and across numerous countries, is crucial.
Resources: Running a patient board calls for considerable investment, including internal expertise, time, and funds. Resources need to be anticipated and allocated upstream. Having a stable leadership team contributes considerably to the buy-in of participants, builds trust, and contributes to the sustainability of projects.
Defining value: It is a challenge to translate such a collaboration into tangible value or measurable impact in a company’s R&D processes, both short-term and long-term. Since a patient board may not be directly linked to a therapeutic product or to specific projects, staff may need to extend themselves beyond their normal responsibilities, to stay motivated and engaged.
Applying and adapting tools end-to-end, from project administration, and monitoring, to evaluation: It is advisable for companies to select carefully and upstream from reputable guidelines, checklists, and tools, including monitoring and evaluation frameworks, and to use them systematically to ensure a full end-to-end quality assessment, including post-project results [33–35].
Challenges, lessons learned, and recommendations for the broader community
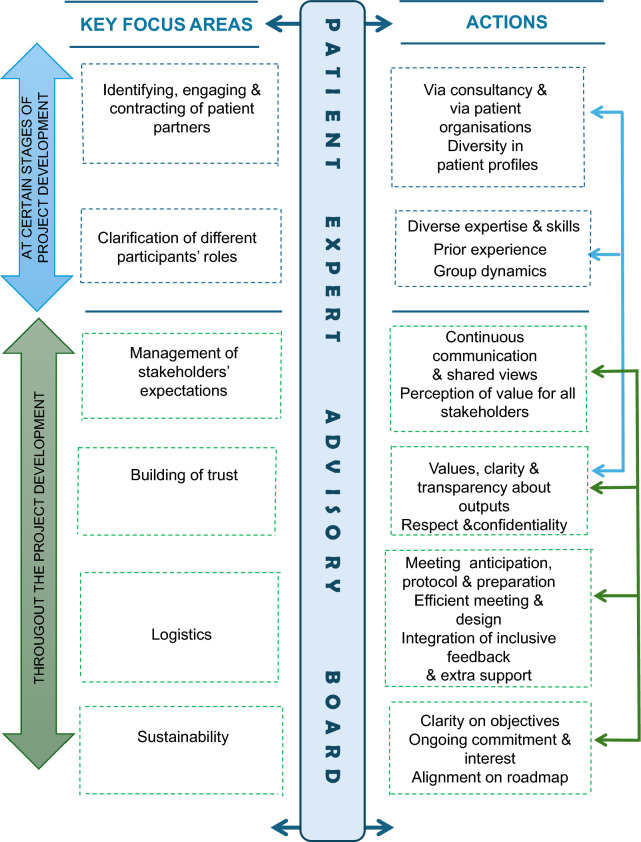
In addition to these unique industry-specific challenges, more general challenges and solutions were noted in the experience of creating a patient board. Table 3 provides this broader overview of challenges, both relating to technical and adaptive skills. It provides some examples that emerged through the process and suggests potential solutions. Figure 4 provides a visual synopsis of these key focus areas, challenges, and corresponding actions.
Table 3.
Establishing and running a patient board: Key focus areas, challenges, and recommendations
| Key focus areas | Challenges | Recommendations |
|---|---|---|
| Identifying patient partners |
The company was seeking patient partners with prior experience in working with R&D and/or research projects Finding patient partners with a suitable level of expertise (Fig. 2) can be challenging |
Consider consulting with global or regional patient organisations, whose members already include patient experts Consider identifying skilled and trained-up patient partners via a patient consultancy |
| Aiming for group diversity in patient engagement |
Networks of patient partners may lack diversity, of all kinds The company used three Europe-wide channels to recruit patient experts. Although the patient board was ultimately diverse in certain aspects (age, gender, disease type, geography, profiles, and types of personalities), the networks used did not attract applicants of racial/ethnic diversity |
Consider preparing a clear and comprehensive diversity strategy upfront and building full diversity into recruitment criteria Be mindful that having a mix of personality types, diverse profiles, and different cultures or countries of origin is likely to foster more enriching dialogue and varied group dynamics |
| Recruiting and contracting patient partners | The company aimed to recruit and remunerate patient partners according to fair market value, while adhering to the compliance and legal frameworks of different countries | Consider seeking advice from a patient-founded consultancy that can advise on fair and legally robust practices, potentially acting as a third-party facilitator |
| Building trust among all members of the group |
Building trust in the process of collaboration requires time, resources, and careful, sustained communication. Patient partners and company employees may have not worked closely in such a setting before Patient partners may be wary of tokenism [45]: feeling invited to contribute, but not having their perspectives acknowledged or taken on board |
It is advisable to build on established, reputable frameworks of high ethical standards, including integrity and respect (Fig. 3), and to refine them together Aim to make the intentions of all participants transparent, to guide the collaboration as it develops It is advisable for those involved in creating and running a patient board to communicate clearly throughout the collaboration about the concrete deliverables or outputs, as well as their anticipated meaning and impact |
| Managing expectations vs feasibility | Expectations from patient partners may be difficult to balance with the constraints of the life sciences industry, especially regarding the extent to which patient partners can take consultative or decision-making roles |
As above, those involved in creating a patient board would ideally communicate throughout the collaboration about the concrete deliverables or outputs, as well as their anticipated meaning and impact When recommendations from patient board members cannot be implemented, allow an honest and frank discussion about why not, with opportunities to explore whether other solutions with similar outcomes may be available Promoting a culture of transparency regarding the collaboration is beneficial for all, patient partners and company employees Managing expectations from both patient board members and company staff is important to sustainability |
| Managing expectations and commitment in long-term R&D projects |
R&D takes time, and progressing from research through preclinical and development phases may span 12–20 years This time, combined with high attrition rates (when drug candidates are withdrawn), makes it difficult to maintain interest from patient partners and to sustain relationships |
In addition to the suggestions above, be transparent about the uncertainties of the projects Co-create and define a format of communication to maintain the quality of the relationship between meetings Address potential reluctance felt by company staff by discussing with them the challenges of engaging with patient partners on long-term R&D projects |
| Logistics: Planning across country calendars and many agendas | Identifying dates that are convenient for a large group, living in different countries with different work schedules and holiday calendars, is challenging |
Plan as far in advance as possible, offering calendar polls to identify the best possible dates, with maximum attendance Take into account time zones, when organising meeting times Consider recording meetings, so those who cannot attend can watch a recording afterwards Discuss and agree on what counts as the minimum acceptable number of board members attending per meeting |
| Logistics: Patient fatigue during long meetings |
Some board members suffered fatigue and difficulty concentrating through longer meetings Patient partners may need extra support to fully participate as members of a group [45]. Challenges may include timing and pace of meetings and the number of items in a meeting agenda |
Consider holding meetings of an agreed-upon maximum duration (e.g. two hours), building in breaks that are more frequent and longer than might otherwise be proposed For this collaboration, meetings were made shorter, and more frequent, longer breaks were introduced |
| Logistics: Technological challenges | Some patient partners struggled with new online technology (notably online brainstorming platforms). They may live with conditions that limit their exposure to certain digital tools |
Consider building in pre-meeting time to run tech checks and/or pre-meeting training sessions Consider adapting digital tools to participants’ digital literacy or applying them in a simplified format; provide extra training and support, if required Consider using polls and online voting as a user-friendly way to engage with patient partners |
| Logistics: Communication difficulties |
Some board members had difficulty with speech, relating to their diseases Similarly, participants may have varying levels of skill and confidence in the given working language of the meetings |
Consider accepting feedback, both verbal and via typed chat functions, from participants In this case, feedback from all channels was welcome, during and after meetings, and integrated into meeting reports Consider emerging digital communication tools, such as simultaneous interpretation or artificial intelligence transcript generation, enabling participants to follow meetings better |
| Logistics: Balancing in-person vs online meetings |
Not meeting in person can make forging relationships difficult. Several board members only met in person for the first time at the end of the ideas-generation period Some patient partners have difficulties travelling or with their mobility; they appreciate a balance of in-person and online meetings |
While online meetings support equity of access of patient partners, it can be beneficial for group members to meet in person Consider holding an in-person meeting early in the process to allow relationships to form and flourish Consult patient partners about their needs and constraints, such as relating to travel, accommodation, or diet |
| Sustainability: Managing the collaboration to its closure |
It can be challenging to maintain the interest and commitment of patient partners in a collaboration, for which consultations vary in frequency and according to the project’s stage and development A lack of clear and formal closure to a project (or phases of it) can lead to participants feeling poorly informed or even misled |
Consider defining clear objectives, with a roadmap, showing dates, goals and actions, from start to finish. Continuity may be applicable only to certain smaller tasks, as the project draws to its close Remind participants where the project started, where it has led, and if possible, how close it is to the end At the end, ensure a summary meeting is held and closure is clear to all Consider how the project’s sustainability will be linked to the company’s longer-term interest, buy-in, and commitment of resources |
Fig. 4.
Key focus areas and actions for creating a patient board. Note Available for reproduction under a Creative Commons License CC BY-NC-SA 4.0
Conclusion
This case study presents a constructive, practical, and positive example of patient engagement within a life sciences company, ahead of the launch of its R&D institute, shaping a more patient-centric company culture. It highlights a notable gap, as there are relatively few such peer-reviewed, published case studies in this area to date. Via a patient board, it is possible to bring together patient experts to engage meaningfully, purposefully, and impactfully with the life sciences industry, creating and working towards shared goals, within the legal and compliance regulations that the life sciences industry faces. We aim to offer readers valuable insights into the establishing and running of such a patient expert board, and the impacts such an approach may create.
Such a process, however, is not without challenges, including those challenges unique to the company, and those which can be more broadly applied to patient engagement collaborations. Over a two-year, organic, ideas-generation phase of this board, experience and reflection led to many learnings, with ongoing adaptations and actions continuing to emerge. A summary of considerations, lessons learned, approaches applied, and resulting recommendations may serve as a model to be used–and adapted–by companies or organisations seeking to engage with patients in a meaningful, and non-tokenistic way. We encourage others to plan well ahead to strive for diversity in their patient engagement work, to build trust and transparency with care, to seek a balance between expectations and feasibility, to devote attention to sustainability, and to never underestimate the importance of preparation and practical considerations.
The life sciences industry stands at a transformative crossroads. Going forward, it can transcend its traditional product-centric model and embrace a patient-centric ethos that incorporates elements of the voices, values, and needs of patients. That this is such uncharted territory for many makes it a ripe opportunity to share experiences, learn, and build new ways. We look forward to seeing more such studies published, incorporating assessment of how similar engagement affects the product-centric model, its sustainability, and the evolution of measurable company strategies, structures, and practices.
We hope that this case study will be one of many to come, in which companies or organisations can, by sharing experiences and learnings, forge new ground and develop stronger patient-centric practices in healthcare settings and drug development.
Supplementary Information
Acknowledgements
With their consent, we wish to acknowledge and thank all who contributed to this case study. Patient experts who have served on the Servier Saclay R&D Patient Expert Board were: Veerle Aertsen; Ana Amariutei; Richard Ballerand; Koen Block; Graham Brown; Catherine Cerisey; Marc van Grieken; Sabrina Grigolo; Tamara Hussong Milagre; Estelle Jobson; Robert Joyce; Anita Kienesberger; Begoña Nafría Escalera; Thomas Smith; Oriana Sousa; Gilliosa Spurrier; Linda Stone; and Janet West. Tamás Bereczky provided valuable input; and additionally for their guidance, support, and project management, we express our warm thanks to Patvocates staff, Jan Geissler, Hamda Munawar, and Linda Silva. Many members of the Servier staff also supported and participated in this collaboration.
Abbreviations
- DCT
Decentralised trial
- EMA
European Medicines Agency
- EUPATI
European Patients’ Academy on Therapeutic Innovation
- FDA
U.S. Food and Drug Administration
- PFMD
Patient Focused Medicines Development
- PRO
Patient-reported outcomes
- PXD
Patient experience data
- R&D
Research and development
- UK
United Kingdom
- US
United States
- WHO
World Health Organization
Author contributions
Corresponding authors (EJ and MG): Estelle Jobson: EJ’s contributions to the manuscript were: Original draft, Substantial contributions, Data analysis, Data collection, Literature review, Writing and revision, Preparing figures and tables, Copy-editing and proofreading, and approved the submitted version and is/are accountable for their own contributions. Marta Garcia: MG’s contributions to the manuscript were: Original draft, Substantial contributions, Data analysis, Data collection, Literature review, Writing and revision, Preparing figures and tables and approved the submitted version and is accountable for their own contributions. Life sciences company authors (LR, SA, AL): Laura Risueño: LR’s contributions to the manuscript were: Original draft, Substantial contribution, Preparing figures and tables, Literature review, Writing and revision, and approved the submitted version and is accountable for their own contributions. Sylvain Arnould: SA’s contributions to the manuscript were: Original draft, Substantial contribution, Literature review, Writing and revision and approved the submitted version and is accountable for their own contributions. Aude Lemoine-André: AL’s contributions to the manuscript were: Original draft and approved the submitted version and is accountable for their own contributions. Patient consultancy authors (DS and JG): Danika Sharek: DS’s contributions to the manuscript were: Original draft, Substantial contributions, Literature review, Writing and revision and approved the submitted version and is accountable for their own contributions. Jan Geissler: JG’s contributions to the manuscript were: Revision and approved the submitted version and is accountable for their own contributions. Patient co-authors (AA, SG, BN, TS, OS, LS, JW) Ana Amariutei: AA's contributions to the manuscript were: Revision, Proofreading and approved the submitted version and is accountable for their own contributions. Sabrina Grigolo: SG’s contributions to the manuscript were: Revision, Suggesting peer reviewer and approved the submitted version and is accountable for their own contributions. Begonya Nafria Escalera: BN’s contributions to the manuscript were: Revision, Proofreading and approved the submitted version and is accountable for their own contributions. Thomas Smith: TS’s contributions to the manuscript were: Revision, Proofreading and approved the submitted version and is accountable for their own contributions. Oriana Sousa: OS’s contributions to the manuscript were: Revision, Suggesting peer reviewer, Proofreading and approved the submitted version and is accountable for their own contributions. Linda Stone: LS’s contributions to the manuscript were: Revision and approved the submitted version and is accountable for their own contributions. Janet West: JW’s contributions to the manuscript were: Revision, Proofreading and approved the submitted version and is accountable for their own contributions.
Funding
This study was sponsored by Servier.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethical approval and consent to participate
Not applicable. This manuscript reports author experiences of a retrospective case study; no data collection was conducted as part of this case study. As such, ethical approval and consent to participate were not required. All authors of this study were members of the patient board described in the research or employees of the life sciences company, and no external participants were involved.
Consent for publication
Not applicable. All authors consent to be named accordingly. All people thanked in the Acknowledgements section consent to being acknowledged, too.
Competing interests
Some authors (SA, AL, LR, and GM) are full-time employees of Servier. No authors own Servier stocks. One author (JG) is CEO of the patient consultancy, Patvocates. One author (DS) is a part-time employee of Patvocates. One author (AA) is both a full-time employee of Patvocates and a member of the patient board. The co-corresponding author (EJ), and patient contributors (AA, SG, BN, TS, OS, LS, JW) are members of the Servier Saclay R&D Patient Expert Board, as described in the article, and their affiliation appears accordingly.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Estelle Jobson, Email: estelle.jobson@gmail.com.
Marta Garcia, Email: marta.garcia@servier.com.
References
- 1.Taylor K. Paternalism, participation and partnership—the evolution of patient centeredness in the consultation. Patient Educ Couns. 2009;74:150–5. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Constitution of the World Health Organization [Internet]. WHO; 1948 [cited 2024 Feb 1]. Available from: https://www.who.int/about/accountability/governance/constitution
- 3.Stergiopoulos S, Michaels DL, Kunz BL, Getz KA. Measuring the impact of patient engagement and patient centricity in clinical research and development. Ther Innov Regul Sci. 2020;54:103–16. 10.1007/s43441-019-00034-0. [DOI] [PubMed] [Google Scholar]
- 4.U.S. FDA PEAC. Patient Engagement in Medical Device Clinical Trials: Discussion Document [Internet]. U.S. FDA; 2018 [cited 2024 Feb 15]. Available from: https://www.fda.gov/media/117890/download
- 5.U.S. FDA. Patient Engagement in the Design and Conduct of Medical Device Clinical Studies: Guidance for Industry, Food and Drug Administration Staff, and Other Stakeholders [Internet]. Patient Engagem. Des. Conduct Med. Device Clin. Stud. FDA; 2022 [cited 2024 Feb 15]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-engagement-design-and-conduct-medical-device-clinical-studies
- 6.U.S. Food and Drug Administration. FDA Patient-Focused Drug Development Guidance Series for Enhancing the Incorporation of the Patient’s Voice in Medical Product Development and Regulatory Decision Making (Four Guidances) [Internet]. FDA. 2024 [cited 2024 Feb 1]. Available from: https://www.fda.gov/drugs/development-approval-process-drugs/fda-patient-focused-drug-development-guidance-series-enhancing-incorporation-patients-voice-medical
- 7.U.S. FDA. Patient-Focused Drug Development: Collecting Comprehensive and Representative Input [Internet]. FDA; 2018 Jun. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-focused-drug-development-collecting-comprehensive-and-representative-input
- 8.U.S. Food and Drug Administration. Benefit-Risk Assessment for New Drug and Biological Products: Guidance for Industry [Internet]. U.S. FDA; 2023 Oct. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/benefit-risk-assessment-new-drug-and-biological-products
- 9.CIOMS Working Group XI on Patient involvement in the development, regulation and safe use of medicines. Patient involvement in the development, regulation and safe use of medicines [Internet]. Council for International Organizations of Medical Sciences (CIOMS); 2022. Available from: https://cioms.ch/publications/product/patient-involvement/
- 10.European Medicines Agency. Principles on the involvement of young patients/consumers within EMA activities [Internet]. 2017 May. Report No.: EMA/494077/2016. Available from: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/principles-involvement-young-patientsconsumers-within-ema-activities_en.pdf
- 11.European Medicines Agency. Engagement Framework: EMA and patients, consumers and their organisations [Internet]. EMA; 2022 [cited 2024 Feb 1]. Available from: https://www.ema.europa.eu/system/files/documents/other/updated_engagement_framework_-_ema_and_patients_consumers_and_their_organisations_2022-en.pdf
- 12.Bertelsen N, Dewulf L, Ferrè S, Vermeulen R, Schroeder K, Gatellier L, et al. Patient engagement and patient experience data in regulatory review and health technology assessment: a global landscape review. Ther Innov Regul Sci. 2024;58:63–78. 10.1007/s43441-023-00573-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James J. Health policy brief: patient engagement. Health Aff. 2013. 10.1377/hpb20130214.898775/full/healthpolicybrief_86.pdf. [Google Scholar]
- 14.Wong-Rieger D. Moving from patient advocacy to partnership: a long and bumpy road. Patient Patient-Centered Outcomes Res. 2017;10:271–6. 10.1007/s40271-017-0216-1. [DOI] [PubMed] [Google Scholar]
- 15.Marie-Pascale Pomey and Paule Lebel. Patient Engagement: The Quebec Path*. Healthc Pap [Internet]. 2016;16:80–5. Available from: https://www.longwoods.com/content/24998/patient-engagement-the-quebec-path- [PubMed]
- 16.Vargas C, Whelan J, Brimblecombe J, Allender S. Co-creation, co-design, co-production for public health – a perspective on definition and distinctions. Public Health Res Pract. 2022. 10.17061/phrp3222211. [DOI] [PubMed] [Google Scholar]
- 17.Scilife. Life Sciences Industry: Complete Definition and Examples [Internet]. Life Sci. Ind. Complete Defin. Ex. 2023 [cited 2024 Feb 1]. Available from: https://www.scilife.io/glossary/life-science
- 18.Yeoman G, Furlong P, Seres M, Binder H, Chung H, Garzya V, et al. Defining patient centricity with patients for patients and caregivers: a collaborative endeavour. BMJ Innov. 2017;3:76–83. 10.1136/bmjinnov-2016-000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson H, Dashiell-Aje E, Anatchkova M, Coyne K, Hareendran A, Leidy NK, et al. Beyond study participants: a framework for engaging patients in the selection or development of clinical outcome assessments for evaluating the benefits of treatment in medical product development. Qual Life Res. 2018;27:5–16. 10.1007/s11136-017-1577-6. [DOI] [PubMed] [Google Scholar]
- 20.Weston AD, Hood L. Systems biology, proteomics, and the future of health care: toward predictive, preventative, and personalized medicine. J Proteome Res. 2004;3:179–96. 10.1021/pr0499693. [DOI] [PubMed] [Google Scholar]
- 21.Boffetta P, Collatuzzo G. Application of P4 (predictive, preventive, personalized, participatory) approach to occupational medicine. Med Lav Work Environ Health. 2022;113:e2022009. 10.2349/mdl.v113i1.12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorbenko O, Cavillon P, Giles RH, Kolarova T, Marks M, Cardone A, et al. Co-creating with patients an impact framework across the medicine’s life cycle: a qualitative study exploring patients’ experiences of involvement in and perceptions of impact measures. Res Involv Engagem. 2022;8:1. 10.1186/s40900-022-00334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauman AE, Fardy HJ, Harris PG. Getting it right: why bother with patient-centred care? Med J Aust. 2003;179:253–6. 10.5694/j.1326-5377.2003.tb05532.x. [DOI] [PubMed] [Google Scholar]
- 24.Domecq JP, Prutsky G, Elraiyah T, Wang Z, Nabhan M, Shippee N, et al. Patient engagement in research: a systematic review. BMC Health Serv Res. 2014;14:89. 10.1186/1472-6963-14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malfait S, Van Hecke A, De Bodt G, Palsterman N, Eeckloo K. Patient and public involvement in hospital policy-making: Identifying key elements for effective participation. Health Policy. 2018;122:380–8. [DOI] [PubMed] [Google Scholar]
- 26.Ryll B. 12/52: The difference between patients and patient advocates [Internet]. 1252 Differ. Patients Patient Advocates. 2022 [cited 2024 Feb 11]. Available from: https://www.mpneurope.org/post/12-52-the-difference-between-patients-and-patient-advocates
- 27.National Institute for Health and Care Research (NIHR). A brief guide to public involvement in funding applications [Internet]. Brief Guide Public Involv. Funding Appl. 2020 [cited 2024 Feb 1]. Available from: https://www.nihr.ac.uk/documents/a-brief-guide-to-public-involvement-in-funding-applications/24162
- 28.Auwal FI, Copeland C, Clark EJ, Naraynassamy C, McClelland GR. A systematic review of models of patient engagement in the development and life cycle management of medicines. Drug Discov Today. 2023;28:103702. [DOI] [PubMed] [Google Scholar]
- 29.Levitan B, Getz K, Eisenstein EL, Goldberg M, Harker M, Hesterlee S, et al. Assessing the financial value of patient engagement: a quantitative approach from CTTI’s patient groups and clinical trials project. Ther Innov Regul Sci. 2018;52:220–9. 10.1177/2168479017716715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klingmann I, Heckenberg A, Warner K, Haerry D, Hunter A, May M, et al. EUPATI and patients in medicines research and development: guidance for patient involvement in ethical review of clinical trials. Front Med. 2018;5:251. 10.3389/fmed.2018.00251/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Preston J, Biglino G, Harbottle V, Dalrymple E, Stalford H, Beresford MW. Reporting involvement activities with children and young people in paediatric research: a framework analysis. Res Involv Engagem. 2023;9:61. 10.1186/s40900-023-00477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dews S-A, Bassi A, Buckland S, Clements L, Daley R, Davies A, et al. Characterising meaningful patient and public involvement in the pharmaceutical industry research setting: a retrospective quality assessment. BMJ Open. 2023;13:e071339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vat LE, Finlay T, Robinson P, Barbareschi G, Boudes M, Diaz Ponce AM, et al. Evaluation of patient engagement in medicine development: a multi-stakeholder framework with metrics. Health Expect. 2021;24:491–506. 10.1111/hex.13191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fruytier S, Vat LE, Camp R, Houÿez F, Keyser HD, Dunne D, et al. Monitoring and evaluation of patient engagement in health product research and development: co-creating a framework for community advisory boards. J Patient-Centered Res Rev. 2022;9:46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.L’Espérance A, O’Brien N, Grégoire A, Abelson J, Canfield C, Del Grande C, et al. Developing a Canadian evaluation framework for patient and public engagement in research: study protocol. Res Involv Engagem. 2021;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Servier. Annual Report 2020/2021 [Internet]. Available from: https://servier.com/wp-content/uploads/2022/09/servier-annual-report-2020-21.pdf
- 37.Anderson M, Kimberly MK. On the path to a science of patient input. Sci Transl Med. 2016;8:336ps11. 10.1126/scitranslmed.aaf6730. [DOI] [PubMed] [Google Scholar]
- 38.Clinical Trials Transformation Initiative (CTTI). Presenting CTTI Recommendations: Effective Engagement with Patient Groups Around Clinical Trials [Internet]. CTTI. 2015 [cited 2024 Feb 1]. Available from: https://ctti-clinicaltrials.org/presenting-ctti-recommendations-effective-engagement-with-patient-groups-around-clinical-trials/
- 39.Patvocates. About Patvocates: Who we are [Internet]. 2023 [cited 2024 Feb 1]. Available from: https://www.patvocates.net/about-patvocates/
- 40.Innovative Medicines Initiative (IMI). IMI Innovative Medicines Initiative [Internet]. Eur. Patients Acad. Ther. Innov. Summ. 2017 [cited 2024 Feb 1]. Available from: http://www.imi.europa.eu/projects-results/project-factsheets/eupati
- 41.EUPATI. Email correspondence: Number of qualified EUPATI Fellows. 2023.
- 42.European Patients’ Academy on Therapeutic Innovation. EUPATI Annual Report 2023. 2024 [cited 2024 Feb 15]; Available from: https://eupati.eu/wp-content/uploads/2024/02/EUPATI-Annual-Report-2023-7.pdf
- 43.Patient Focused Medicines Development. Patient Engagement Quality Guidance v 2.0 [Internet]. PFMD; 2018 [cited 2024 Feb 1]. Available from: https://patientfocusedmedicine.org/peqg/patient-engagement-quality-guidance.pdf
- 44.Deane K, Delbecque L, Gorbenko O, Hamoir AM, Hoos A, Nafria B, et al. Co-creation of patient engagement quality guidance for medicines development: an international multistakeholder initiative. BMJ Innov. 2019. 10.1136/bmjinnov-2018-000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richards DP, Cobey KD, Proulx L, Dawson S, de Wit M, Toupin-April K. Identifying potential barriers and solutions to patient partner compensation (payment) in research. Res Involv Engagem. 2022;8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Servier, BioLabs Global. Press release: Servier and BioLabs call for applications to join Spartners, the integrated incubator within the future Servier Research and Development Institute in Paris-Saclay [Internet]. Servier and BioLabs Global, media departments; 2022 [cited 2024 Feb 1]. Available from: https://servier.com/wp-content/uploads/2022/10/servier-biolabs-application-spartners_PR.pdf
- 47.Servier. Servier Integrated Annual Report 2021/2022 [Internet]. Available from: https://servier.com/wp-content/uploads/2023/03/servier-integrated-annual-report-2021-22.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.