Figure 1. ABCA1 stably expressed in HEK cells is functionally active.
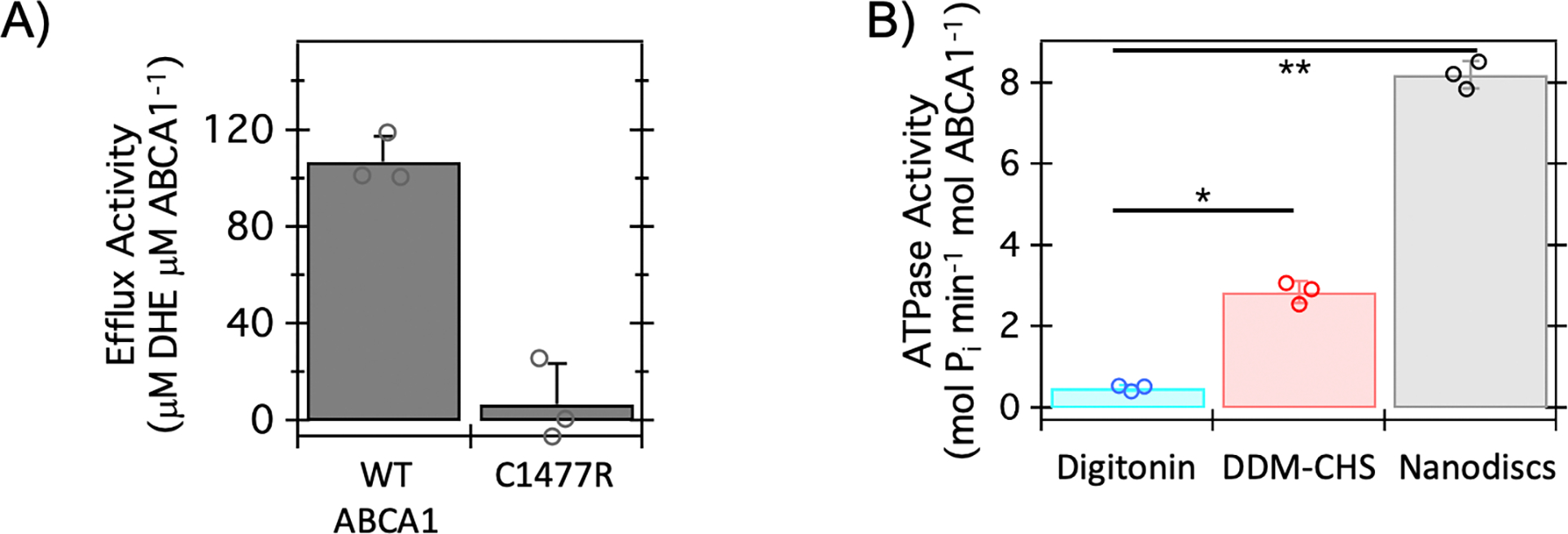
A) Cell-based efflux activity of ABCA1 for the cholesterol analog, dehydroergosterol (DHE) is shown for wild-type (WT) ABCA1 and the well-characterized ECD mutant: C1477R. Modulating the ECD conformation (i.e., C1477R) reduces the measured sterol efflux of ABCA1. Data represent the average of three biological replicate experiments and error bars indicate the standard deviation; individual data points are shown. The probability associated with a Student’s paired t-test with a two-tailed distribution for comparison of these data is < 0.025. Briefly, DHE efflux was monitored in HEK Expi293F cells stably expressing WT-NGFP-ABCA1 or C1477R with the addition of exogeneous His-apoA-I. B) ATPase activity of ABCA1 was measured in two detergents: digitonin and Dodecyl-β-Maltoside with cholesteryl hemisuccinate (DDM-CHS), along with nanodiscs prepared with 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1’-rac-glycerol) (POPG) lipid and the membrane scaffold protein (MSP) E3D1. Data represent the average of three biological replicate experiments and error bars indicate the standard deviation; individual data points are indicated. The probability associated with a Student’s paired t-test with a two-tailed distribution for data demarcated by a single asterisk (*) is < 0.0025. The probability associated with a Student’s paired t-test with a two-tailed distribution for data demarcated by a double asterisk (**) is < 0.0005. Briefly, WT-ABCA1 was purified from HEK Expi293F cells and isolated through affinity chromatography in the respective detergents or reconstituted into nanodiscs containing POPG lipids and the Membrane Scaffold Protein (MSP) E3D1 after cleavage of the N-terminal GFP. ATP hydrolysis was quantified by colorimetric assay of phosphate release.
