Abstract
Background:
Longitudinal trends in per- and polyfluoroalkyl substances (PFAS) serum concentrations across pregnancy have not been thoroughly examined, despite evidence linking prenatal PFAS exposures with adverse birth outcomes.
Objectives:
We sought to characterize longitudinal PFAS concentrations across pregnancy and to examine the maternal–fetal transfer ratio among participants in a study of risk and protective factors for adverse birth outcomes among African Americans.
Methods:
In the Atlanta African American Maternal–Child cohort (2014–2020), we quantified serum concentrations of four PFAS in 376 participants and an additional eight PFAS in a subset of 301 participants during early (8–14 weeks gestation) and late pregnancy (24–30 weeks gestation). Among these, PFAS concentrations were also measured among 199 newborns with available dried blood spot (DBS) samples. We characterized the patterns, variability, and associations in PFAS concentrations at different time points across pregnancy using intraclass correlation coefficients (ICCs), maternal–newborn pairs transfer ratios, linear mixed effect models, and multivariable linear regression, adjusting for socioeconomic and prenatal predictors.
Results:
Perfluorohexane sulfonic acid (PFHxS), perfluorooctane sulfonic acid (PFOS), perfluorooctanoic acid (PFOA), and perfluorononanoic acid (PFNA) were detected in of maternal samples, with PFHxS and PFOS having the highest median concentrations. We observed high variability in PFAS concentrations across pregnancy time points (). All median PFAS concentrations increased from early to late pregnancy, except for PFOA and N-methyl perfluorooctane sulfonamido acetic acid (NMFOSAA), which decreased [paired -test for all PFAS except for PFOA and perfluorobutane sulfonic acid (PFBS)]. Prenatal serum PFAS were weakly to moderately correlated with newborn DBS PFAS ( ). The median maternal–fetal PFAS transfer ratio was lower for PFAS with longer carbon chains. After adjusting for socioeconomic and prenatal predictors, in linear mixed effect models, the adjusted mean PFAS concentrations significantly increased during pregnancy, except for PFOA. In multivariable linear regression, PFAS concentrations in early pregnancy significantly predicted the PFAS concentrations in late pregnancy and in newborns.
Discussion:
We found that the concentrations of most PFAS increased during pregnancy, and the magnitude of variability differed by individual PFAS. Future studies are needed to understand the influence of within-person PFAS variability during and after pregnancy on birth outcomes. https://doi.org/10.1289/EHP14334
Introduction
Per- and polyfluoroalkyl substances (PFAS) are a group of synthetic chemicals widely used in industrial and commercial applications and in a variety of consumer products, including nonstick cookware and food containers, owing to their oil- and water-repellant properties.1–4 PFAS are persistent in the environment and ubiquitous in the US population owing to their long biological half-lives and bioaccumulation.5,6 Because of the health concerns related to PFAS exposures, major global manufacturers announced their voluntary phase-out of new production of perfluorooctanoic acid (PFOA) in 2006 and of perfluorooctane sulfonic acid (PFOS) between 2000 and 2002.3 Nevertheless, these common long-chain PFAS have been replaced with structurally similar, unknown PFAS.3,7 Although both environmental and human concentrations of PFOS8 have gradually declined, these legacy PFAS are still found in environmental media, including food, drinking water, soil, and house dust,9,10 resulting in continued exposure of the general population to these compounds.
Consistently, studies of the US population have shown that almost all individuals have detectable levels of multiple PFAS.8,11 PFAS can readily cross the placental barrier, directly implicating the risk of PFAS exposure to the developing fetus. Notably, concentrations of PFAS in cord blood are often found to be generally comparable or lower than those in maternal blood,12–14 though their presence still poses significant risks to the developing fetus. Epidemiological evidence over the last decade has demonstrated that exposure to PFAS can be a potential risk factor for adverse health outcomes, including pregnancy complications and adverse birth and child health outcomes, as well as neurodevelopmental effects of children.15–19 PFAS exposure during pregnancy, infancy, and early childhood is particularly of interest because these life stages are marked by rapid growth and development, making them more susceptible to PFAS exposures and adverse health risks.4 Thus, investigating the changes in PFAS exposure levels throughout pregnancy and their placental transfer mechanisms is crucial for identifying critical exposure periods and guiding public health policies to reduce PFAS exposure, especially in vulnerable populations.
Despite the epidemiological evidence, information on longitudinal trends of PFAS during pregnancy is limited. Recent longitudinal analysis of PFAS concentrations, measured during pregnancy, postpartum period, and early childhood, showed a decreasing trajectory over time.20–26 In addition, as reported by Oh et al.,21 changes in maternal PFAS concentrations may differ not only across pregnancy and postnatal periods but also in individual PFAS types. Previous studies also identified several important predictors of maternal PFAS exposure during pregnancy, including some sociodemographic, diet, and perinatal predictors.22,27–31 However, none of those studies focused on pregnant African American people, who experience high environmental exposures and high rates of health disparities.32 Overall, findings in previous studies are inconsistent, and much less is known about PFAS concentrations in newborns, which have been predominantly assessed through cord blood samples.20,22,25,26 A limited number of studies have used dried blood spots (DBS) for PFAS measurement on newborns,33–36 and the use of DBS has shown reliability and reproducibility as a minimally invasive and novel approach for population-level studies.
To address these knowledge gaps, we conducted the present study to characterize the patterns and variability of maternal serum PFAS concentrations across early and late pregnancy, as well as PFAS concentrations in newborn DBS at delivery, in the Atlanta African American (AA) Maternal–Child Cohort (2014–2020).
Methods
Study Population and Data Collection
Participants included in this analysis were recruited between 2014 and 2020 as a part of the ongoing prospective Atlanta AA Maternal–Child Cohort, a well-established birth cohort that has been described thoroughly elsewhere.37,38 The cohort was designed as an intra-race study of risk and protective factors for adverse birth outcomes among African Americans in accordance with the March of Dimes Research Agenda,39 which calls for cohort studies to investigate the social, biological, and environmental exposures uniquely affecting African American families due to historical and current inequities. Initial input from focus groups, consisting of pregnant African American patients and health care practitioners, guided the study’s design, particularly in restricting the cohort to US-born African Americans and identifying critical exposure domains, such as psychosocial stress, nutrition, and health behaviors.37
Briefly, pregnant people were recruited from prenatal clinics affiliated with Emory Midtown Hospital (privately funded) and Grady Hospital (publicly funded) if they were between 18 and 40 years of age, able to communicate in English, self-identified as African American or Black race, pregnant with a singleton, and without a diagnosis of chronic medical conditions or chronic use of a prescription medication. This study was approved by the Emory University Internal Review Board, and informed consent was obtained from all participants.
Data collection occurred at early pregnancy (8–14 weeks gestation) and late pregnancy (24–30 weeks gestation) and included a questionnaire on basic demographic information, medical records abstraction for clinical conditions, and venous blood samples for maternal PFAS measurements.29 Detailed characteristics collected included maternal age at study entry, marital status, type of health insurance, poverty-to-income ratio, educational attainment, parity, body mass index (BMI) based on clinically measured weight (in kilograms) and height (in meters squared) at the first visit, and self-reported substance use during the month before the enrollment visit.
DBS were collected from newborns within 24–48 h after birth from a heel stick performed by trained hospital nursery personnel using a standardized protocol.40 Briefly, the skin of the heel was cleaned with 75% isopropanol and a sterile 2.5-mm lancet was used to obtain the sample, which was collected onto a standard Guthrie card by saturating each circle with of blood.41 On the day of collection, card specimens were transported to the Georgia Department of Public Health Laboratory for storage in a walk-in refrigerator (2–8°C) without a desiccant for up to 3 months and then transported to an Emory University Laboratory for storage in a freezer () within gas-impermeable PFAS-free bags with desiccant until assay.41
A total of 638 participants enrolled in the Atlanta AA Maternal–Child Cohort between 2014 and 2020. Among them, 533 participants were tested for at least one type of PFAS during early or late pregnancy. In early pregnancy, 525 maternal serum samples were analyzed for the determination of perfluorohexane sulfonic acid (PFHxS), PFOS, PFOA, and perfluorononanoic acid (PFNA) concentrations, and a subset of 429 maternal serum samples were analyzed to quantify perfluorobutane sulfonic acid (PFBS), perfluoroheptanoic acid (PFHpA), perfluorodecanoic acid (PFDA), perfluoroundecanoic acid (PFUnDA), perfluorododecanoic acid (PFDoDA), perfluorooctanesulfonamide (PFOSA), -methyl perfluorooctane sulfonamido acetic acid (NMFOSAA), and perfluorohexanoic acid (PFHxA); In late pregnancy, 383 maternal serum samples (not entirely included in the 525 and 429 samples) were analyzed to quantify the above 12 PFAS, resulting in 376 having paired PFAS measurements for PFHxS, PFOS, PFOA, and PFNA, and 301 having paired PFAS measurements for the additional 8 PFAS in both early and late pregnancy. Meanwhile, a subset of 280 newborns’ DBS samples were analyzed for PFHxS, PFOS, PFOA, and PFNA, resulting in 199 maternal–newborn dyads. The participants were selected based on the availability of their serum samples for assays, prioritizing the first consecutively enrolled participants with sufficient serum volume to measure the 4 main PFAS: PFHxS, PFOS, PFOA, and PFNA. PFAS measurements were completed under different grant mechanisms, which required the use of two different laboratories, one of which completed a 4-panel PFAS and the other that completed a 12-panel PFAS, further causing interlaboratory differences in the number of measurements between each lab. The detailed sample size illustration of each PFAS type is shown in Figure 1.
Figure 1.
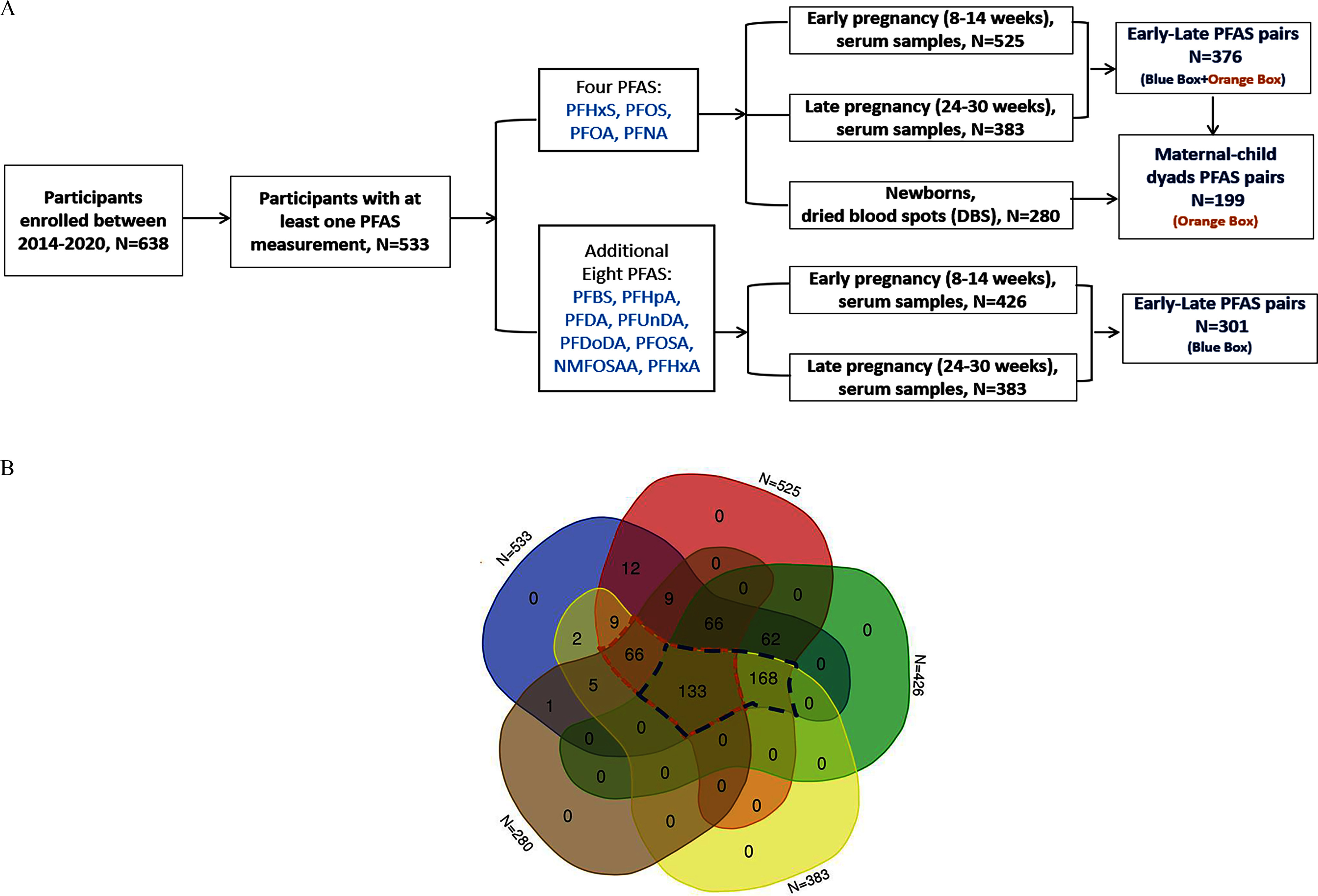
(A) Flowchart of participants with PFAS samples at different time points and (B) the overlap between different sample sizes from the Atlanta African American Maternal–Child Cohort included in our analytic samples, 2014–2020. The orange/blue box notes indicate the size of the paired samples: early-late four PFAS pairs: ; early-late eight PFAS pairs: ; maternal–newborn dyads: . Among participants, 2 participants were missing maternal demographic information. The demographic statistics Table S4 was thus based on participants. was used in the intraclass correlation coefficients (ICCs), transfer ratio, Spearman correlation among maternal–newborn dyads, linear mixed effect models, and multivariable linear regression. was used in the generalized additive models, ICCs, and Spearman correlation among maternal pairs. Note: NMFOSAA, -methyl perfluorooctane sulfonamido acetic acid; PFAS, per- and polyfluoroalkyl substances; PFBS, perfluorobutanesulfonic acid; PFDA, perfluorodecanoic acid; PFDoDA, perfluorododecanoic acid; PFHpA, perfluoroheptanoic acid; PFHxA, perfluorohexanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; PFOSA, perfluorooctanesulfonamide; PFUnDA, perfluoroundecanoic acid.
PFAS Analysis
PFAS measurement in maternal serum samples.
Maternal venous blood samples collected during pregnancy were centrifuged to obtain serum and stored at . The maternal serum samples in early pregnancy were analyzed in two laboratories, namely Children’s Health Exposure Analysis Resource (CHEAR) at and the Laboratory of Exposure Assessment and Development for Environmental Health Research (LEADER), both at Emory University. Maternal samples in late pregnancy and newborn DBS were analyzed by the Wadsworth Center Human Health Exposure Analysis Resource (HHEAR). CHEAR and HHEAR are exposure assessment consortia of the National Institute of Environmental Health Studies and the core laboratories of CHEAR/HHEAR implemented harmonized quality control procedures for delivering comparable data.42 Further details of the analytical methods for PFAS quantification have been described elsewhere.29,43 Briefly, samples were spiked with internal standards, then extracted by solid phase extraction (SPE), and subsequently analyzed by liquid chromatography–tandem mass spectrometry (LC-MS/MS). The isotope-dilution method was used to quantify PFAS concentration with the limits of detection (LODs) ranging from 0.05 to . The LODs of PFAS measurement in each laboratory for both maternal and newborn samples are provided in Table S1. Excellent agreement was found between the results of quality control/duplicate samples analyzed from the two laboratories for maternal samples (Table S2).29
NY Wadsworth Laboratory.
A hybrid SPE (Hybrid-SPE) and ultrahigh-performance LC-MS/MS (UPLC-MS/MS) were used in the analysis of 13 PFAS in maternal serum at the Wadsworth lab. Serum PFAS were extracted using a Hybrid-SPE–phospholipid cartridge after precipitating proteins and other endogenous biological interferences with 1% ammonium formate in methanol (MeOH). Two hundred fifty microliters of serum was transferred to a polypropylene (PP) tube and spiked with of an isotope-labeled internal standard mixture ( of each compound). To this mixture, of MeOH containing 1% ammonium formate (wt/vol) was added, and the mixture was shaken for 30 s. The supernatant was separated by centrifugation at for 5 min at room temperature using a centrifuge (Eppendorf 5804; Barkhausenweg). Hybrid-SPE cartridges were prewashed with of MeOH containing 1% ammonium formate (wt/vol) to remove any contamination that might arise from the cartridge. The supernatant was quantitatively transferred to the cartridge and the eluate was collected and transferred in a vial for instrumental analysis. The chromatographic separation was carried out using UPLC (Acquity I Class; Waters) coupled with an electrospray ionization triple-quadrupole mass spectrometer (ESI-TQMS; API 5500; AB SCIEX). The target analytes were separated by an Acquity UPLC BEH C18 (, ) column (Waters). The mobile phase for the UPLC comprised 100% MeOH for mobile phase A and 0.1% ammonium acetate in water for mobile phase B. The flow rate was set at min-1 starting at 10% MeOH, held for 0.75 min, increased to 70% over 0.25 min and then to 75% over 1 min, increased to 100% over 1.2 min and held for 0.9 min, decreased to 10% over 0.1 min and held for 0.8 min (for a total run time of 5 min). Injection volume was . Quantification of PFAS was based on the isotope-dilution method, and target analytes were monitored by multiple reaction monitoring (MRM) mode under negative ionization.
Procedural blanks were analyzed with each batch of 20 samples. Quantification was by isotope dilution. The average intraday accuracy (measured as percentage recoveries from fortified samples) and precision of the method [measured as percentage relative standard deviation (RSD) between analyses] were 88.7%–117% and 1.0%–13.4%, respectively. The average interday precision was 2.8%–6.9%. The method was sensitive with the limits of quantification (LOQs) in the range of 0.05 to for all 13 PFAS. The laboratory regularly participates in external quality assessment schemes to validate the method. National Institute of Standards and Technology (NIST) Standard Reference Materials (SRMs) were analyzed with each batch of samples, and the values were within the reference ranges.
Emory LEADER Laboratory.
For this study, four PFAS were measured in human serum using online SPE coupled with LC-MS/MS. Serum aliquots () were spiked with isotopically labeled internal standards and then mixed. Six hundred microliters of formic acid was added to each sample and mixed continuously for 5 min at and then sonicated for 20 min. Samples were transferred to autosampler vials and injected into a column switching system for isolation and concentration of the target analytes on a solid SPE analytical column. The online SPE-high-performance LC (HPLC) was performed using an Agilent 1260 Infinity system (Agilent Technologies) consisting of two binary pumps, two degassers, an autosampler with a temperature control module set at 8°C, and a temperature-stable column compartment. An external 10-port switching valve (1290 Valve Drive; Agilent Technologies) was used to facilitate column switching. The samples were eluted by mobile phase flow reversal onto a BetaSil C18 analytical column (, ) for chromatographic separation using a gradient elution beginning at 70% mobile phase A ( ammonium acetate, pH 4.0) and ending with 100% mobile phase B (acetonitrile) before reequilibrating at 70% mobile phase A. PFAS and their labeled analogs were analyzed using MRM on an Agilent 6400 series TQMS (Agilent Technologies) equipped with an atmospheric pressure ESI interface in the negative ion mode. One quantification ion and one confirmation ion were monitored for the native compounds, and one quantification ion was monitored for the labeled analogs. Concentrations of the target compounds were determined using isotope-dilution calibration, which is calculated from the relative response (per volume of sample injected) of the native-to-labeled standards in the samples relative to the matrix-matched standard calibration curve. In each analytical run, samples were analyzed concurrently with an 11-point calibration curve, 1 serum and 1 reagent blank sample, 2 quality control samples (at a high and a low concentration), and a NIST SRM. The average interday accuracy (measured from NIST SRM samples) and RSDs ranged from 89.2% to 115% and 2.2% to 11.8%, respectively. The average interday RSD ranged from 1.5% to 7.2%. The method was sensitive, with LOQs in the range of 0.08– for all PFAS.
PFAS measurement in DBS samples.
The method for the analysis of four PFAS in newborn DBS has been described in detail elsewhere.34,44 Briefly, a diameter punch from DBS, estimated to contain of whole blood, was used for analysis. The volume of whole blood spotted on the Guthrie card from heel sticks can vary, and we estimated the volume based on an empirical measure36 and some level of uncertainty is associated with this estimate.45 Analytes were identified and quantified using an Agilent 1100 series HPLC coupled with a SCIEX 5500 ESI-TQMS.
DBS samples were cut into small pieces (using solvent-cleaned scissors) and placed in a PP tube. Two milliliters of Milli-Q water and 13C-labeled internal standards [PFOA, PFNA, PFOS and PFHxS ( each)] were added to each sample and sonicated for 60 min. Then, the solution was extracted by an ion-pair extraction method by adding of tetrabutyl ammonium hydrogen sulfate solution (adjusted to pH 10), of sodium carbonate/sodium bicarbonate buffer, and of methyl tert-butyl ether (MTBE). After shaking for 40 min in an orbital shaker (Eberbach) at 250 oscillations/min, the MTBE layer was separated by centrifugation (Eppendorf Centrifuge 5804) at for 5 min. The MTBE layer was transferred into a new PP tube, evaporated to dryness under a gentle stream of nitrogen gas, and reconstituted with of MeOH. After vortexing, the extract was transferred into a gas chromatography vial for HPLC-MS/MS analysis. An ABSciex API 5500 ESI-TQMS (Applied Biosystems), coupled with an Agilent 1100 series HPLC system (Agilent Technologies Inc.), was used for identification and quantification of the target chemicals. Chromatographic separation was carried out on a Betasil C18 column (, ; Thermo Electron Corporation) connected serially with a Javelin guard column (Betasil C18, , ; Thermo Electron Corporation). The mobile phases (flow rate: ) were MeOH and ammonium acetate water (, ammonium acetate dissolved in MeOH and Milli-Q water) at a gradient starting from 20% MeOH, increased linearly to 100% MeOH at 4 min (held for 4 min), then decreased to 20% MeOH (held for 5 min). Five microliters of the extract were injected, and a negative ionization MRM mode was used for all target chemicals. Data collection and analysis were performed by Analyst software (version 1.6; Applied Biosystems).
Quality assurance and quality control protocols included analysis of blank spots from each sample to account for background levels of contamination on the DBS card, analysis of procedural blanks to account for background levels of contamination arising from laboratory reagents and solvents, and analysis of matrix spikes. Recoveries of target analytes through the method were between 80% and 120%. RSDs of the repeated analysis were .
Statistical Analysis
Descriptive analyses were conducted for 12 PFAS in maternal serum samples and 4 PFAS in newborn DBS, including detection frequency, geometric means (GMs), geometric SDs (GSDs), and percentiles. PFAS with low detection frequencies (0%–40%) in both maternal samples included PFHpA, PFDoDA, PFOSA, and PFHxA and were excluded from further analysis. PFAS levels below the LODs were imputed as the LOD divided by the square root of 2.46 After visual inspection of distributions of the data, PFAS concentrations were natural log-transformed to reduce the effect of potential outliers. Spearman correlation coefficients of PFAS concentrations were calculated to examine the correlation of PFAS between paired maternal samples and paired maternal–newborn samples.
For comparison of whole blood PFAS measured in newborn DBS with maternal serum concentrations, a factor of 2 was applied, based on a previous study that reported the serum-to-whole blood ratio for PFAS was .47 Thus, to normalize whole blood levels measured in newborn DBS to serum levels measured in pregnant people, we doubled the PFAS concentration in newborn DBS. Previous studies have reported excellent agreement of concentrations in finger prick DBS samples and venous whole blood samples.35,48
Intraclass correlation coefficients (ICCs) and 95% confidence intervals (CIs) were computed to assess the within-participant variability of natural log-transformed PFAS concentrations across maternal pairs and between maternal–newborn pairs. The paired -test was performed to assess whether the increase or decrease of PFAS across the pregnancy was significant. Generalized additive models (GAMs) were used to visualize the PFAS concentrations across gestational weeks [shrinkage cubic regression spline in GAM, formula = y ∼ s(x, bs = “cs”)]. Maternal–newborn transfer ratio was calculated through Equation 1:
| (1) |
where CDBS is the doubled PFAS concentration in newborn DBS (in nanograms per milliliter), and CME and CML are the PFAS concentrations in maternal early and late serum samples (in nanograms per milliliter), respectively. is the transfer ratio from PFAS in maternal early samples to PFAS in DBS, and is the transfer ratio from PFAS in maternal late samples to PFAS in DBS.
Then, we fitted a covariate-adjusted linear mixed effect model with a random intercept for each maternal–newborn dyad identifier to assess the change in PFAS concentrations across different sample periods (maternal serum samples in early and late pregnancy and newborn DBSs) using Equation 2, where i represents each participant and j represents the three sample periods. The following covariates were selected based on our previous analysis29 and an analysis of variance test (Table S3), including maternal age (continuous), married or cohabiting (yes, no), education (less than high school, high school, some college, college and above), hospital site (Emory, Grady), parity (0, 1, ), prenatal BMI (continuous), sex of infant (male, female), alcohol use and marijuana use 1 month prior to pregnancy (yes, no). The directed acyclic graph (https://www.dagitty.net/) for hypothesized relationships between repeated maternal serum and newborn DBS PFAS are shown in Figure S1.
| (2) |
In addition, to simultaneously assess the adjusted correlation between maternal and newborn PFAS concentrations and examine the effect of socioeconomic and prenatal factors on PFAS concentrations, we employed multivariable linear regression. Specifically, we fitted two sets of models: a) to explore the effect of maternal PFAS levels in early pregnancy, along with socioeconomic and prenatal factors, on PFAS concentrations in late pregnancy (maternal PFAS during late pregnancy as dependent variables and maternal PFAS during early pregnancy as predictors with covariates); b) to determine the effect of PFAS concentrations during early and late pregnancy, as well as the important predictors, on PFAS concentrations in newborn DBS (newborn DBS PFAS as dependent variables and maternal early and late PFAS as predictors with covariates, respectively).
| (3) |
All main analyses were run on complete cases, with no missing data. For sensitivity analysis, to reduce potential selection bias and residual confounding attributable to the data availability on the small subset of maternal–newborn dyads () relative to the overall study population (), we created inverse probability weights. To do this, we first used logistic regression to predict the probability of selection for each participant within the maternal–newborn pairs (the propensity scores). The propensity models were conditional on the same set of covariates as Equation 2. We also identified the area of common support, which is defined as the range between the minimum propensity score among participants within the maternal–newborn dyads and among participants not within the maternal–newborn dyads.49 Only those participants whose propensity scores fell within the area of common support were included in the further analyses. Then, the inverse probability weights of each participant within the area of common support were calculated based on Equation 3, where treatment refers to the variable indicating whether participants were within the maternal–newborn dyads. Finally, we fitted the adjusted linear mixed effect model with these inverse probability weights to assess the unbiased adjusted percentage change of PFAS over pregnancy.
For all models in the analysis, PFAS concentrations were natural log-transformed for a better fit. All statistical analyses were performed using R (version 4.2.0; R Core Development Team).
Results
Descriptive Analysis
The sociodemographic and behavioral characteristics of participants with paired PFAS measurements available in both early and late pregnancy and mother–newborn dyads are shown in Table 1. The overall study population of the present study included pregnant people and their newborns with at least one PFAS measurement available (, two participants were demographic information) (Table S4). There were no statistically significant differences () in the distribution of characteristics when comparing the overall study population to the three sample subsets. Overall, the mean maternal age at enrollment was 25.08 y, and the average BMI was . More than half of the participants had a high school or below education (54.6%), income-to-poverty ratio (66.3%), had Medicaid as medical insurance (78.9%), and had given birth at least once (53.3%).
Table 1.
Sociodemographic and clinical characteristics [ or (%)] of study participants by different PFAS pairs in the Atlanta African American Maternal–Child cohort, 2014–2020.
| Characteristics | Paired maternal () | Paired maternal () | Paired newborn () |
|---|---|---|---|
| Maternal age (y) | |||
| Married or cohabiting | |||
| Yes | 180 (47.9) | 145 (48.2) | 84 (42.2) |
| No | 195 (51.9) | 156 (51.8) | 115 (57.8) |
| Maternal education level | |||
| Less than high school | 55 (14.6) | 44 (14.6) | 29 (14.6) |
| High school | 145 (38.6) | 115 (38.2) | 84 (42.2) |
| Some college | 110 (29.3) | 89 (29.6) | 53 (26.6) |
| College graduate and above | 65 (17.3) | 53 (17.6) | 33 (16.6) |
| Income-to-poverty ratio | |||
| 167 (44.4) | 130 (43.2) | 88 (44.2) | |
| to | 79 (21.0) | 66 (21.9) | 42 (21.1) |
| to | 75 (20.0) | 68 (22.6) | 42 (21.1) |
| 54 (14.4) | 37 (12.3) | 27 (13.5) | |
| Health insurance | |||
| Private | 80 (21.3) | 60 (19.9) | 51 (25.6) |
| Medicaid | 295 (78.4) | 241 (80.1) | 164 (74.4) |
| Hospital site | |||
| Grady Memorial Hospital | 225 (59.8) | 181 (60.1) | 121 (60.8) |
| Emory University Hospital–Midtown | 150 (39.9) | 120 (39.9) | 78 (39.2) |
| Alcohol use in month prior to pregnancy | |||
| No | 340 (90.4) | 273 (90.7) | 184 (92.5) |
| Yes | 35 (9.3) | 28 (9.3) | 15 (7.5) |
| Tobacco use in month prior to pregnancy | |||
| No | 320 (85.1) | 260 (86.4) | 179 (89.9) |
| Yes | 55 (14.6) | 41 (13.6) | 20 (10.1) |
| Marijuana use in month prior to pregnancy | |||
| No | 243 (64.6) | 194 (64.5) | 125 (62.8) |
| Yes | 132 (35.1) | 107 (35.5) | 74 (37.2) |
| Gestational age at first sample collection | |||
| Gestational age at second sample collection | |||
| Body mass index () | |||
| Parity (number of births) | |||
| 0 | 161 (42.8) | 134 (44.5) | 79 (39.7) |
| 1 | 108 (28.7) | 85 (28.2) | 54 (27.1) |
| 106 (28.2) | 82 (27.3) | 66 (33.2) | |
| Sex of infant | |||
| Female | 195 (51.9) | 157 (52.2) | 103 (51.8) |
| Male | 180 (47.9) | 144 (47.8) | 96 (48.2) |
Note: PFAS pairs (): subset to PFHxS, PFOS, PFOA, and PFNA in maternal early and late sample pairs (), with one participant missing demographic information; PFAS pairs (): subset to additional eight PFAS in maternal early and late sample pairs; PFAS pairs (): subset to PFHxS, PFOS, PFOA, and PFNA maternal and newborn pairs. PFAS, per- and polyfluoroalkyl substances; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; SD, standard deviation.
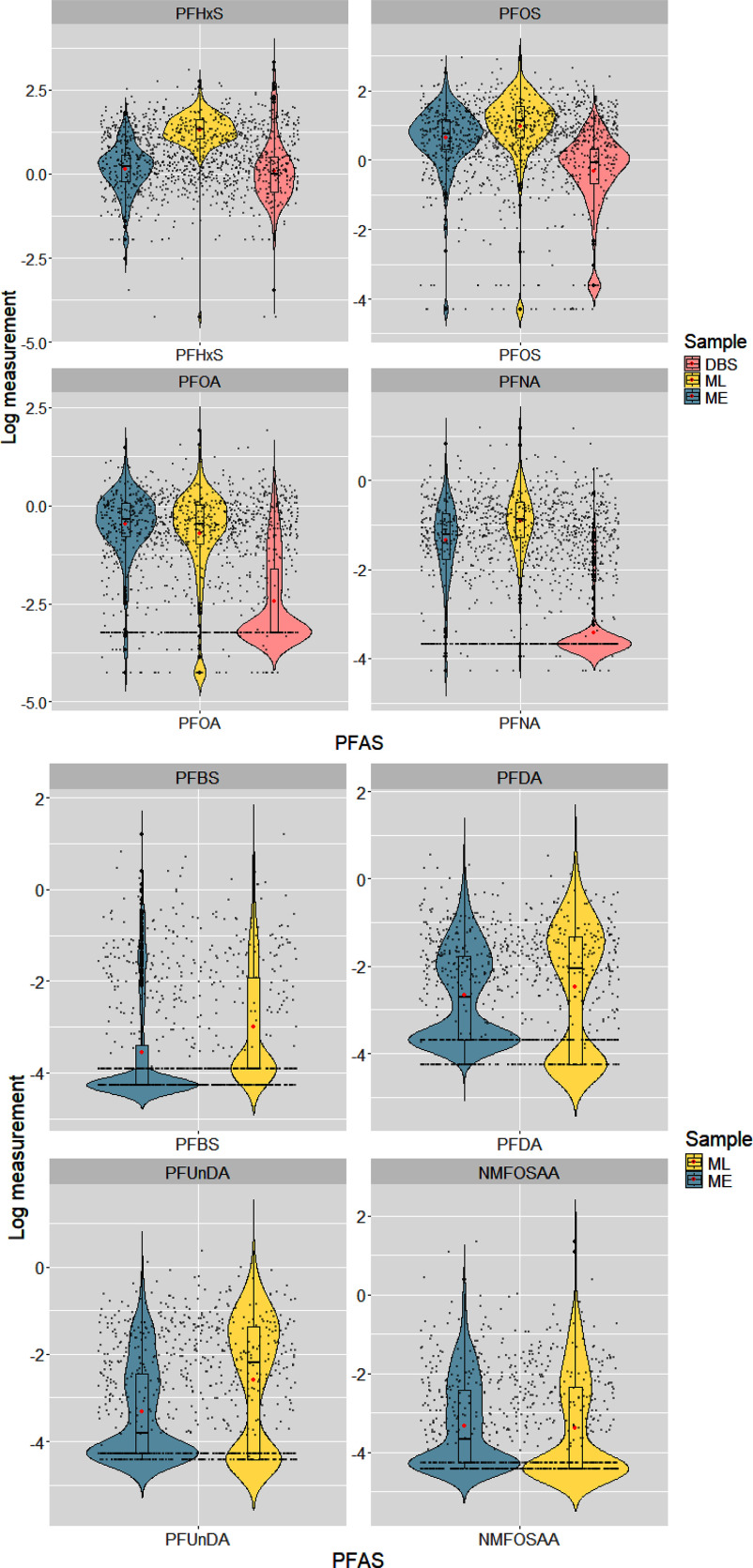
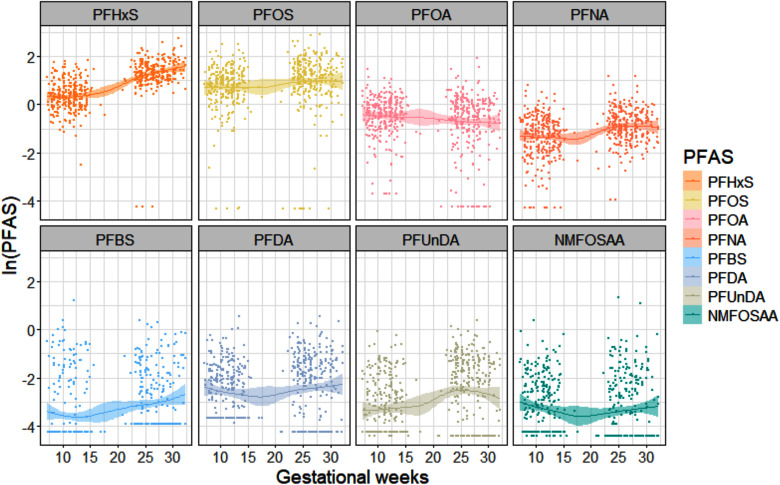
PFAS concentrations in maternal serum samples and newborn DBS are summarized in Table 2. PFHxS, PFOS, PFOA, and PFNA were detected in of the maternal samples, with the highest GM of for PFHxS, followed by for PFOS, in maternal late pregnancy. Generally, PFAS have lower GM in early pregnancy samples (ranging from 0.04 to ) than late pregnancy samples (ranging from 0.03 to ), except for PFOA and NMFOSAA. When restricting to participants with paired samples, the distribution of PFAS concentrations was similarly higher in late pregnancy compared with early pregnancy. Moreover, following normalization of PFAS concentration in measured DBS to serum measures, PFAS concentrations were lower in newborns than in pregnant women (Figure 2). We found an increasing trend for PFHxS, PFOS, PFNA, PFBS, PFDA, and PFUnDA concentrations and a decreasing trend for PFOA and NMFOSAA concentrations across pregnancy (Figure 3). After conducting the paired -test between maternal PFAS pairs, we found all PFAS significantly changed over time (), except for PFOA () and PFBS ().
Table 2.
Distribution of PFAS concentrations () in maternal serum and newborn dried blood spot samples collected from participants in the Atlanta African American Maternal–Child cohort, 2014–2020.
| PFAS | Sample | Detection (%) | Percentile | |||||
|---|---|---|---|---|---|---|---|---|
| 25th | 50th | 75th | Max | |||||
| PFHxS | Maternal early | 525 | 98 | 0.81 | 1.24 | 1.74 | 6.17 | |
| Maternal late | 383 | 99 | 2.88 | 3.79 | 5.01 | 15.6 | ||
| Newborn | 280 | 99 | 0.29 | 0.51 | 0.84 | 13.9 | ||
| PFOS | Maternal early | 525 | 98 | 1.38 | 2.12 | 3.24 | 12.4 | |
| Maternal late | 383 | 97 | 1.92 | 3.11 | 4.69 | 19.2 | ||
| Newborn | 280 | 94 | 0.26 | 0.47 | 0.69 | 2.07 | ||
| PFOA | Maternal early | 525 | 97 | 0.45 | 0.70 | 1.06 | 4.42 | |
| Maternal late | 383 | 94 | 0.37 | 0.63 | 1.01 | 6.76 | ||
| Newborn | 280 | 41 | 0.10 | 1.02 | ||||
| PFNA | Maternal early | 525 | 98 | 0.17 | 0.30 | 0.47 | 2.27 | |
| Maternal late | 383 | 99 | 0.28 | 0.42 | 0.61 | 3.25 | ||
| Newborn | 280 | 13 | 0.37 | |||||
| PFBS | Maternal early | 429 | 28 | 0.03 | 3.32 | |||
| Maternal late | 383 | 43 | 0.14 | 2.29 | ||||
| PFHpA | Maternal early | 429 | 19 | 0.92 | ||||
| Maternal late | 383 | 44 | 0.22 | 6.45 | ||||
| PFDA | Maternal early | 429 | 57 | 0.07 | 0.17 | 1.73 | ||
| Maternal late | 383 | 67 | 0.13 | 0.27 | 1.69 | |||
| PFUnDA | Maternal early | 429 | 51 | 0.02 | 0.09 | 0.93 | ||
| Maternal late | 383 | 68 | 0.11 | 0.25 | 1.44 | |||
| PFDoDA | Maternal early | 429 | 8 | 0.63 | ||||
| Maternal late | 383 | 20 | 3.46 | |||||
| PFOSA | Maternal early | 429 | 3 | 0.18 | ||||
| Maternal late | 383 | 2 | 0.07 | |||||
| NMFOSAA | Maternal early | 429 | 53 | 0.03 | 0.09 | 1.46 | ||
| Maternal late | 383 | 47 | 0.10 | 3.83 | ||||
| PFHxA | Maternal early | 429 | 10 | 1.16 | ||||
| Maternal late | 383 | 37 | 0.11 | 3.53 | ||||
Note: GM, geometric mean; GSD, geometric standard deviation; LOD, limit of detection; max, maximum; NMFOSAA, -methyl perfluorooctane sulfonamido acetic acid; PFAS, per- and polyfluoroalkyl substances; PFBS, perfluorobutanesulfonic acid; PFDA, perfluorodecanoic acid; PFDoDA, perfluorododecanoic acid; PFHpA, perfluoroheptanoic acid; PFHxA, perfluorohexanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; PFOSA, perfluorooctanesulfonamide; PFUnDA, perfluoroundecanoic acid.
Figure 2.
The distribution of natural log-transformed PFAS across maternal serum samples and newborn dried blood spots among study participants with paired samples available within the Atlanta African American Maternal–Child cohort, 2014–2020 ( (left side) for mother–newborn dyads and (right side) for maternal early and late sample pairs). PFAS in DBS were normalized to serum concentrations for comparison with maternal serum PFAS. Medians and interquartile ranges are shown in the center box plots, and arithmetic means are indicated by red dots. Note: DBS, dried blood spots; ME, maternal early samples; ML, maternal late samples; NMFOSAA, -methyl perfluorooctane sulfonamido acetic acid; PFAS, per- and polyfluoroalkyl substances; PFBS, perfluorobutanesulfonic acid; PFDA, perfluorodecanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; PFUnDA, perfluoroundecanoic acid.
Figure 3.
Generalized additive models (GAMs) plot of natural log-transformed PFAS (ng/mL) across gestational weeks using maternal serum sample pairs among study participants within the Atlanta African American Maternal–Child cohort, 2014–2020 [ (top row) or (bottom row)]. Note: NMFOSAA, -methyl perfluorooctane sulfonamido acetic acid; PFAS, per- and polyfluoroalkyl substances; PFBS, perfluorobutanesulfonic acid; PFDA, perfluorodecanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; PFUnDA, perfluoroundecanoic acid.
The Spearman correlations between log-transformed PFAS concentrations for early and late pregnancy maternal samples and maternal and newborn DBS are presented in Figure S2 and Table S5. For maternal samples, PFAS concentrations in the same pregnancy stage showed a stronger and mostly positive correlation (first and fourth quadrant) (ranging from to 0.79, with the highest correlation between PFOS and PFNA, followed by PFOA and PFNA) compared with PFAS across the early and late pregnancy stage (second and third quadrant) (ranging from to 0.47) (Figure S2, left; Table S5A). The correlation of PFAS across maternal samples and newborn DBS samples was weak to moderate, with correlation coefficients no larger than 0.6 (Figure S2, right; Table S5B).
Variability Analysis
Maternal–fetal PFAS transfer ratios are summarized in Table 3. The highest median transfer ratio among PFAS was found for PFHxS from maternal early serum () and for PFOS from maternal late serum (), respectively. A descending trend in both and was observed, with increasing carbon chain length from PFHxS (C6) to PFNA (C9). Generally, the median PFAS transfer ratio was higher during early pregnancy, except for PFOA (, ). To exclude the potential effect of extreme values, samples below the LOD in maternal and newborn samples were removed (Figure S3). Approximately 53% and 10% of PFHxS samples had transfer ratios of for and , respectively. A few PFOA (7% in ) and PFOS (14% in and 7% in ) samples also had transfer ratios of (Figure S3).
Table 3.
Summary statistics (%) of PFAS transfer ratio (newborn dried blood spots to maternal early/late serum) in Atlanta African American Maternal–Child cohort, 2014–2020 ().
| Mean | Min | Q1 | Median | Q3 | Max | |
|---|---|---|---|---|---|---|
| Newborn DBS–maternal early serum | ||||||
| PFHxS (C6) | 244 | 4.6 | 41.2 | 91.6 | 223 | 5,422 |
| PFOS (C8) | 194 | 1.1 | 32.0 | 56.3 | 94.1 | 10,291 |
| PFOA (C8) | 24.9 | 2.4 | 6.7 | 11.2 | 31.1 | 158 |
| PFNA (C9) | 22.4 | 3.0 | 7.0 | 11.1 | 22.1 | 262 |
| Newborn DBS–maternal late serum | ||||||
| PFHxS (C6) | 230 | 1.0 | 14.7 | 25.7 | 48.1 | 18,969 |
| PFOS (C8) | 146 | 0.6 | 19.4 | 36.4 | 66.1 | 4,893 |
| PFOA (C8) | 124 | 2.2 | 7.6 | 15.3 | 50.5 | 8,815 |
| PFNA (C9) | 18.0 | 0.8 | 5.8 | 8.3 | 15.5 | 191 |
Note: PFAS in DBS were converted to serum basis, for comparison with maternal serum PFAS. Samples measured below the limit of detection (LOD) were imputed with the LOD divided by the square root of 2. DBS, dried blood spots; max, maximum; min, minimum; PFAS, per- and polyfluoroalkyl substances; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; Q1, first quartile; Q3, third quartile.
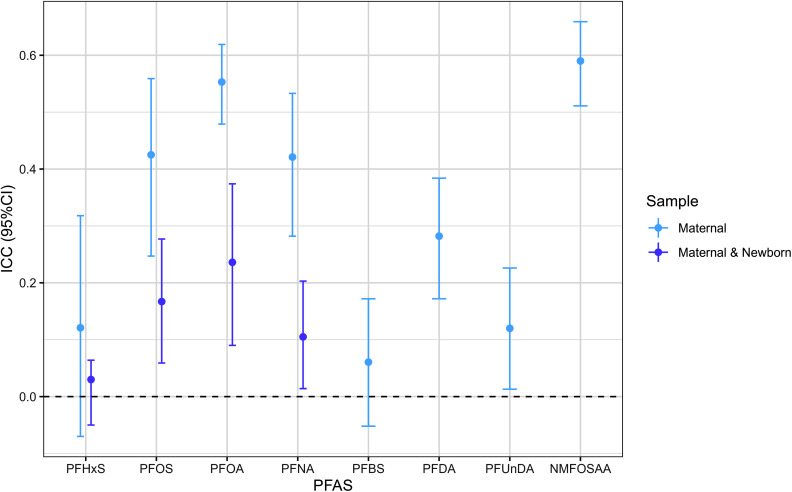
During pregnancy, paired maternal samples exhibited substantial within-participant variation in PFAS, as evidenced by relatively low ICCs [ranging from 0.06 (PFHxS) to 0.59 (NMFOSAA)]. Larger variability was observed for maternal–newborn pairs [ranging from 0.03 (PFHxS) to 0.24 (PFOA)] (Figure 4 and Table S6). PFHxS was most variable in maternal–newborn dyads (), and NMFOSAA was least variable in maternal pairs (). PFOS and PFNA showed similar variability in both maternal pairs and maternal–newborn dyads.
Figure 4.
Intraclass correlation coefficients (ICCs) and 95% CIs of natural log-transformed PFAS concentrations among paired maternal samples [ (PFHxS, PFOS, PFOA, and PFNA) or 301 (PFBS, PFDA, PFUnDA, and NMFOSAA)] and maternal–newborn pairs (). The summary data can be found in Table S4. PFAS in DBS were normalized to serum concentrations for comparison with maternal serum PFAS. Samples measured below the LOD were imputed with the LOD divided by the square root of 2. Note: CI, confidence interval; DBS, dried blood spots; LOD, limit of detection; NMFOSAA, -methyl perfluorooctane sulfonamido acetic acid; PFAS, per- and polyfluoroalkyl substances; PFBS, perfluorobutanesulfonic acid; PFDA, perfluorodecanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; PFUnDA, perfluoroundecanoic acid.
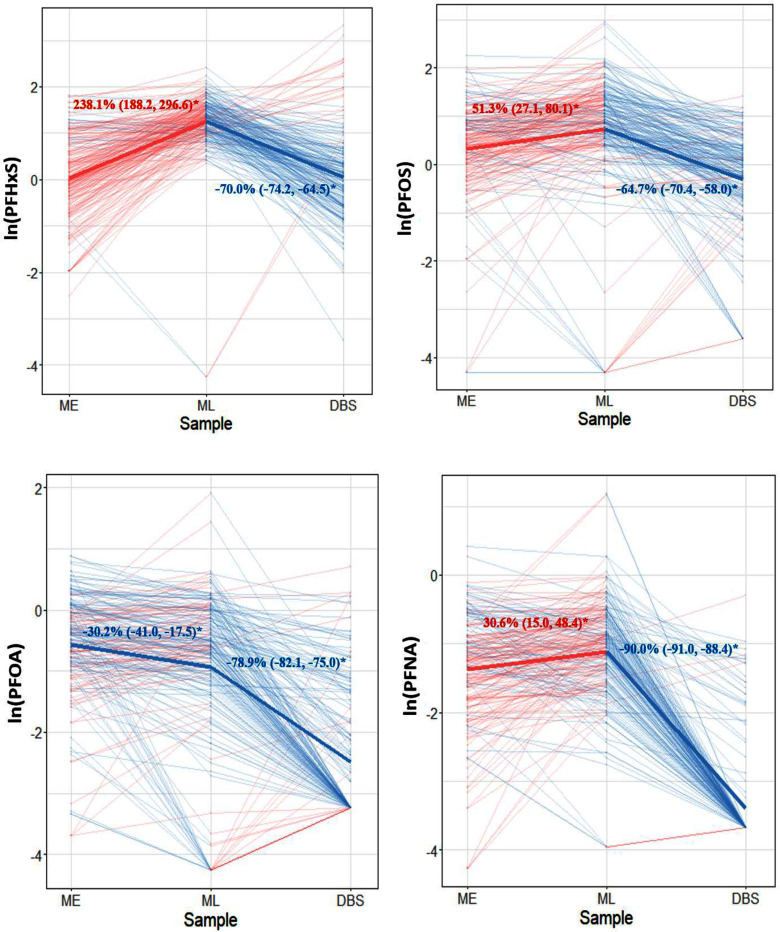
On average, PFHxS, PFOS, and PFNA significantly increased during pregnancy after adjusting for the maternal factors, with the greatest increase of 238.1% for PFHxS (95% CI: 188.2%, 296.6%) and modest increase of 30.6% for PFNA (95% CI: 15.0%, 48.4%), whereas PFOA decreased during pregnancy (95% CI: , ) (Figure 5). The adjusted mean PFAS concentrations were generally lower in newborns at delivery compared with the PFAS concentrations during pregnancy, especially for PFNA, which may be an artifact of imputation for values below LOD. After applying inverse probability weights, the adjusted percentage change of PFAS during pregnancy showed similar results. Still, PFHxS showed the greatest increase from early to late pregnancy [244.6% (95% CI: 221.8%, 265.7%)] and PFOA decreased moderately during pregnancy [ (95% CI: , )] (Figure S4). Meanwhile, the estimated propensity scores within maternal–newborn dyad subsets exhibited distributions similar to those of the remaining subsets from the overall study population, suggesting our analysis pairs were comparable to the overall study population (Figure S4).
Figure 5.
Adjusted percentage change and 95% CIs for PFAS concentrations among maternal–newborn pairs () using linear mixed effect models. PFAS in DBS were normalized to serum concentrations for comparison with maternal serum PFAS. Samples measured below the LOD were imputed with the LOD divided by the square root of 2; models were adjusted for age, education, hospital site, parity, body mass index, alcohol, marijuana use, and infant sex. Thin lines represent individual trajectories of PFAS concentrations. Thick lines represent the adjusted mean concentrations of PFAS on population level for early pregnancy–late pregnancy and late pregnancy–delivery time points. Red and blue color indicate an increased or decreased PFAS, respectively. Statistically significant changes over time are indicated by an asterisk. Note: CI, confidence interval; DBS, dried blood spots; LOD, limit of detection; ME, maternal early samples; ML, maternal late samples; PFAS, per- and polyfluoroalkyl substances; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid.
The associations of sociodemographic and behavioral characteristics with pregnancy and newborn PFAS concentrations are summarized in Tables S7 and S8. PFAS concentrations in early pregnancy significantly predicted PFAS concentrations in late pregnancy for PFHxS, PFOS, PFNA, PFOA, PFDA, PFBS, and NMFOSAA, with the largest association being observed for PFOA. A 1% increase of PFOA in early pregnancy was associated with a 0.63% increase of PFOA in late pregnancy (95% CI: 0.60%, 0.86%) (Table S7). The increase of PFAS concentrations in late pregnancy was also associated with lower maternal age, not being married or cohabiting, higher education levels, and lower prenatal BMI in early pregnancy. Among these predictors, education level explained the largest proportion of concentration of PFAS during late pregnancy, with the largest effect on PFDA in that pregnant individuals with college and above education had 125.01% (95% CI: 8.40%, 367.06%) higher PFDA in late pregnancy compared with those with a less than high school education (Table S7). For newborn PFAS concentrations, both maternal early and late PFOS, PFOA, and PFNA were significant predictors for newborn PFAS. Similarly, education, income-to-poverty ratio, hospital site, parity, and marijuana use prior to pregnancy were found to be significant predictors for the newborn PFAS, with the largest prediction driver being income-to-poverty ratio. Meanwhile, the effect size of maternal PFAS and other covariates in prediction of newborn PFAS concentration was similar in both early and late pregnancy (Table S8).
Discussion
To our knowledge, this is the first study to systematically examine longitudinal PFAS exposure among pregnant African American mothers and newborns. In the present analysis, we assessed the patterns, transfer ratios, and predictors of concentrations of maternal serum PFAS during two gestational time points. Most PFAS concentrations increased across gestation, with the exception of PFOA and NMFOSAA. The greatest variability and maternal–fetal transfer ratio were found in PFHxS during pregnancy. We also found maternal serum PFAS, along with several socioeconomic and prenatal predictors, including maternal age, marital status, education, income, parity, prenatal BMI, and marijuana use, to be important predictors for PFAS change during pregnancy and newborn PFAS concentrations. Taken together, this information can bridge the current research gap on longitudinal PFAS exposure assessment and patterns and identify critical windows of exposure during pregnancy. We anticipate that improved understanding of PFAS changes across pregnancy and neonatal periods will help future studies characterize PFAS exposure for pregnant people and their offspring, especially for those from understudied marginalized populations.
Maternal serum PFAS concentrations in the present study population were lower than concentrations measured in the general US population (i.e., the National Health and Nutrition Examination Survey) and other US birth cohorts, except for PFHxS.22,30,50–54 Previous studies have attributed PFHxS exposure to aqueous film forming foam contamination of drinking water or consumer products, such as carpeting and food packaging,55 rather than other exposure routes, such as seafood consumption.56 Moreover, analyses of dust from cars, workplaces, and homes from different populations across eight countries, including the United States, suggested that dust may be a source of PFHxS exposure, especially in high dust ingestion scenarios,57,58 indicating that pollution and consumer products could be important sources of PFAS exposures in our study population.
A limited number of studies have quantified neonatal PFAS concentrations, and those studies mainly used cord blood samples,59–61 given that the application of DBS for PFAS measurement has been largely uncharacterized.34,35,44,62 Despite the different measurements, we found comparable levels of PFHxS, PFOS, PFOA, and PFNA in newborn DBS as reported in cord blood samples in those studies, suggesting a potential alternative for environmental exposure measurement during critical windows of susceptibility. In general, DBS are much easier to collect than cord blood and do not require the immediate presence of a health care professional at parturition.
Previous studies have reported the longitudinal PFAS profiling during pre- and postnatal periods among mothers and infants, which was conducted in diverse geographical locations and populations, including Sweden, the United States, and China. The decreasing trend of PFAS concentrations over pregnancy has been consistently reported in those studies.20,21,23,24,63–66 However, this trend was not applicable to all PFAS compounds. Two studies21,63 conducted in the United States collected maternal blood samples during the first, second, and third trimesters of pregnancy and found that most of the PFAS concentrations decreased, including PFOS, PFOA, PFNA, and PFDA, whereas PFHxS concentrations remained unchanged over pregnancy. Similarly, Kato et al. collected maternal samples at 16 wk of gestation and at delivery among pregnant people in Ohio,66 and observed decreases in PFOS, PFOA, PFNA, and PFOSA, whereas PFHxS and PFDeA concentrations did not change. However, Chen et al. collected maternal plasma at three trimesters and cord blood at delivery from Chinese pregnant people20 and observed reduced levels of PFOS, PFOA, PFNA, and PFUA but increased levels of PFHxS over pregnancy. PFOA and PFOS had pronounced decreases during pregnancy among pregnant people in Sweden64 and China.65 In the present study, we also found lower levels of PFOA and NMFOSAA in mid-to-late pregnancy compared with early pregnancy. Interestingly, for the remaining PFAS, we observed an increasing trend in concentrations over pregnancy. This was especially apparent for PFHxS, with of participants having elevated PFHxS levels in late pregnancy and showing significant changes even after adjusting for covariates, which indicates potential varying sources of PFAS exposures throughout pregnancy. This can be partly explained by the longer half-life of PFHxS (8.5 y) compared with PFOA (2.3–3.8 y).21,67,68
Prior research conducted within the present African American cohort29 identified several behavioral factors during pregnancy that were correlated with elevated PFAS concentrations in early pregnancy; these included household cleaning practices, primary sources of drinking water, consumption of takeout food, and the use of personal care and cosmetic products. These factors indicate that ongoing exposure to PFAS within this cohort is likely through environmental and personal behavioral pathways. Moreover, the observation of a disproportionately higher increase in PFHxS compared with other PFAS compounds further illustrates the environmental pollution and consumer product sources of PFAS exposure among this African American population. The frequent usage of highly detected PFAS products in the present African American cohort suggests a potential continuing exposure of PFAS over pregnancy and adds emphasis to the importance of targeted interventions to mitigate exposure risk during this vulnerable period of development.
The potential continuing exposure during pregnancy might result in the accumulation of PFAS, which might have obscured any decreases from the effects of expanded plasma volume and placental transfer.69,70 Reports have found that changes in PFAS concentration during pregnancy were related to PFAS structure, glomerular filtration rate (GFR), and serum albumin.71 Normally, GFR and renal clearance increase during pregnancy and may cause a decreasing trend of PFAS. However, our previous study with this cohort showed that the pregnancy-related hemodynamics, as indicated by absolute creatinine concentrations and GFR, do not explain the PFAS change during early and middle pregnancy.72 Meanwhile, serum albumin may affect the transfer of PFAS by serving as a binding protein and thus influencing the PFAS concentrations.71 Further investigations of these physiological marker levels and elimination rates of different PFAS types during pregnancy are needed.
In neonatal DBS, the PFAS concentrations were generally lower than PFAS concentrations in maternal serum. Consistent with our findings, other research groups have reported lower PFAS concentrations in cord blood compared with maternal samples.64,66,73 In addition, positive correlation was observed between PFAS in maternal serum, as well as in newborn DBS, which is in line with other studies having reported the positive correlation between PFAS in maternal serum and in newborn cord serum.30,74–76 For the correlation between maternal samples, however, all correlations were less strong for other PFAS compared with PFHxS, PFOS, PFOA, and PFNA, which may be due to the low levels of these PFAS detected leading to higher analytical uncertainty. We also observed weaker correlations of repeated PFAS measures over pregnancy compared with correlations of different PFAS in the same pregnancy stage, which may be explained by different exposure measurement sources in two pregnant periods and substantial within-women variation as indicated by low ICC values. Similarly, the PFAS in DBS were positively correlated with the PFAS in maternal serum. It is worth noting that stronger positive correlations have been observed in the literature using cord blood samples,23,64,73,77 whereas our study using DBS observed weak-to-moderate correlations. These prior studies reported that samples in the first and second trimesters were slightly less correlated with cord serum than samples in the third trimester, which might be due to a longer time interval before delivery. This may partially explain why we observed the weak-to-moderate correlation between maternal serum PFAS and newborn DBS PFAS. In addition, in the adjusted analysis, maternal serum PFAS was identified as a significant predictor of newborn DBS PFAS, whereas the majority of the variability of newborn PFAS was accounted for by socioeconomic factors, including education and income-to-poverty ratio. Thus, the weak-to-moderate correlations may be indicative of the variability of maternal and newborn PFAS levels between participants, which could be attributed to individual-level social or biological determinants.
Carbon chain length, functional group, and structure of PFAS have been reported to affect the efficiency of PFAS transportation across the placental barrier.78–80 An earlier study suggested that PFAS transfer efficiencies decrease with each increasing unit of carbon chain.81 Similarly, we found maternal–fetal transfer ratios were higher for PFAS with shorter carbon chain (i.e., PFHxS) than for PFAS with longer carbon chain (i.e., PFNA) in both early and late pregnancy. Researchers also reported a U-shape trend66 for PFAS transfer across the placenta with increasing chain length. However, we were unable to validate this pattern owing to the limited range of PFAS types measured.
In addition, studies have suggested that carboxylated PFAS (i.e., PFOA and PFNA) are transported more efficiently than those with sulfonyl groups (i.e., PFHxS and PFOS) owing to the binding affinities specific to albumin.71 In the present study, the low transfer ratio of PFNA may be due to the low detection rate of PFNA among newborn DBS. Transfer ratios of PFOS and PFOA were similar during pregnancy, indicating that chain length, rather than functional group, may potentially contribute more to efficiency of transport from mother to fetus. Our findings also suggest that PFHxS is more efficiently transferred across the placenta than the other PFAS, and correspondingly, the median PFHxS concentration in maternal serum and neonatal DBS samples was higher than in the other PFAS. However, to date, it is unknown whether high maternal PFAS concentrations affect transfer efficiency.82 A high amount of PFAS in maternal serum was transferred to the fetuses during the pregnancy, which consequently led to decreased PFAS concentration in maternal serum compared with that in cord serum, indicating the amplification of PFAS exposure through the placenta when the transfer ratio was .12–14,83 Although to our knowledge, no studies have conducted direct comparisons of transfer ratios derived from DBS and in those obtained from cord serum, a strong correlation between DBS samples and in umbilical cord blood, with high sensitivity and specificity, was reported.84,85 Moreover, it is noteworthy that all those studies on placental transfer ratio used cord blood samples, and we found a comparable level of transfer ratios with those studies,86 and the use of newborn DBS can reflect direct fetal exposures by PFAS crossing the placenta. However, many of those studies comparing matched maternal–cord samples collected both serum and cord blood samples later than 32 wk gestation or at birth,23,80,87 when the hematologic changes during pregnancy have reached maximum status and transfer rates of PFAS are highest.86 This further underscores the necessity for attention to the potentially higher PFAS exposures of newborns in this underrepresented population. Future research should also consider comparative analyses of PFAS concentrations in cord blood/serum and DBS among newborns to further substantiate the utility of DBS as an alternative biological matrix for the comprehensive assessment of maternal–newborn transfer of PFAS.
We found that PFAS concentrations in early pregnancy significantly predicted the concentrations of PFAS in late pregnancy, and PFAS concentrations during pregnancy also significantly predicted the PFAS concentrations in newborns, although these findings explained only of PFAS concentrations in late pregnancy and newborns. The attenuated effect magnitude of these PFAS predictors may potentially be attributed to the concurrent existence of other important prenatal predictors and the continuing PFAS exposure during pregnancy. Meanwhile, several common socioeconomic and prenatal predictors were associated with the concentrations of PFAS in late pregnancy and PFAS concentrations in newborns. Previously, our group reported a similar set of predictors for PFAS concentrations in early pregnancy within a subset of participants enrolled in the Atlanta AA Maternal–Child Cohort.29 Similarly, we found the PFAS concentrations during late pregnancy and among newborns were higher among pregnant people who were younger, married, and more educated and had higher income-to-poverty ratios and lower prenatal BMI and substance use.
Socioeconomic status predictors, such as household income and maternal education, were consistently positive predictors of both maternal PFAS concentrations in cohort studies.22,27,28 Education and income-to-poverty ratio were also considered as the largest drivers for the prediction of maternal and newborn PFAS concentrations in our study. Possible explanations could be that higher-income women with higher education can afford to buy products containing PFAS, which may be more expensive (e.g., impregnated materials; waterproof clothing).27,88 They may also have different diet preferences in favor of a healthier diet, including more fish.28 However, the patterns of change of PFAS concentrations were not consistent across all socioeconomic status predictors in the present study’s results. Furthermore, we observed inconsistent results on PFAS in late pregnancy and BMI. Similarly, there were considerable inconsistencies in the reported association between PFAS concentrations and maternal BMI.66,74,89–91 The possible explanation for these mixed results may be due to the hemodynamics issue or could be influenced by other factors, such as socioeconomic status, food choices, health factors, and geographic location.92 Thus, the etiologies of BMI may affect the change of PFAS concentrations rather than BMI itself. Limited studies have examined the potential socioeconomic status and prenatal predictors on newborn PFAS concentration using cord blood,27,28 and they were found to be consistent with our results using newborn DBS. Additional research on a broader range of prenatal predictors on PFAS concentrations and their temporal variation during pregnancy is warranted. Overall, the determination of these important predictors for maternal and neonatal PFAS concentrations allows for the potential implementation of public health prevention in the future.
We identified new patterns, variability, and important predictors for variability of PFAS using longitudinal profiles for both African American mothers and newborns, not only providing a comprehensive estimate of PFAS exposure levels and depicting a dynamic trend over pregnancy, but also allowing us to suggest potential exposure patterns over the course of pregnancy for future investigations into the health effects of PFAS exposures in other populations. Specifically, the observed elevation in PFAS levels during pregnancy indicated the potential continuing exposure of PFAS through home environment and personal behavioral exposure routes in the present cohort,29 including drinking water and cosmetic product sources. Moreover, the high levels of PFHxS detected in this cohort may reflect environmental pollution and consumer product sources of exposure.55,57,58 At the same time, the high PFHxS transfer ratio found in the present study further underscores the importance of understanding the prenatal exposure to PFAS so as to protect fetal health and development. These findings facilitated the early identification of exposure and enabled the potential implementation of targeted interventions to mitigate the health impacts of PFAS exposure during a critical window of pregnancy among African American people. Meanwhile, newborn DBS in our analyses proved to be a potential alternative for direct fetal exposure measurement compared with cord blood, expanding the methodological options for future investigation in this field. This practical and widely available sample type—DBS—could broaden the applicability of PFAS exposure research across diverse populations.
Despite these strengths, our study was limited in several ways. First, owing to the low detection rate for some PFAS, our analyses mainly focused on four primary PFAS, which limited the comprehensive investigation on replacement PFAS patterns over pregnancy. The heterogeneous measurement sources of PFAS, coupled with large relative differences on same participants, further complicated the interpretation of irregular rising patterns of most PFAS concentrations over pregnancy. Second, we imputed the LOD value based on the LOD divided by the square root of 2 instead of imputing a censored lognormal and assigning a random form. This may have led to a nonnormal distribution of some PFAS even after transformation because of the low detection rate. However, given that our PFAS were measured from different labs, which had several LOD values for each PFAS, further complexity was added to the computation. The correlation between PFAS may be biased downward when using a single value for imputation owing to there being no variance accounted for by the dependent variable. However, for multiple imputation, the association can be completely random, which can also bias the overall correlation downward. In addition, a previous study on PFAS concentrations during early pregnancy in present cohort29 used both simple and multiple imputation methods, and the results were quite similar.
Third, owing to budget constraints, the prioritization of samples from the first consecutively enrolled participants in the present ongoing cohort, coupled with the varied capabilities of PFAS measurement labs, resulted in differing sample sizes for various PFAS measurements across pregnancy stages. The small subset of paired mother–newborn dyad samples may have introduced selection bias into the present study, although the demographic and prenatal characteristics were similar between the paired subsets and the overall population. However, we expect any residual selection bias to be minimal because we applied inverse probability weights to account for selection bias within our smaller subset. Fourth, the concentrations of PFAS in newborn DBS were expressed in nanograms per milliliter, which assumed samples to contain a fixed volume of blood given that there are many uncertainties associated with the volume of blood spotted on DBS. We used as the nominal volume, but there is a high variability of associated with this value. Moreover, the assumption that whole blood PFAS concentrations would be one-half of serum PFAS concentrations was made based on previous studies among adult populations.47,93 Such an assumption should be made with caution given that cellular blood volume differs slightly between infants and adults.34 Thus, owing to the variability in blood volume available in DBS and different hematocrit levels between infants and adults, the data from newborn DBS may be subject to error.
Fifth, the variability between maternal and newborn PFAS can be attributable to many factors, and some important predictors, including diet and behavior or lifestyle predictors,27,31 were not considered in the present study owing to limited data availability. In addition, the limited sample size may have been insufficient to tease out good information on all of the covariates tested in the present analysis, so the results should be interpreted with caution. In addition, we did not have information on potential physiological markers such as albumin, which could have helped us to find out if the rising trend of PFAS concentrations was caused by potential complications or renal impairment. Placental function, transfer efficiency, and different sample types can also affect the variability of PFAS among mothers and newborns, highlighting the complexity of assessing and interpreting PFAS exposure in the maternal–fetal dyad. Meanwhile, to obtain nonbiased assessment of PFAS exposure between individuals, a relatively narrow time window at a particular trimester might be most important.71 Owing to the limited sample size, we did not use trimester-based sample collection, which may have better reflected the trend of exposures. Further studies are needed for more time points during pregnancy. Especially for the transfer ratio in the present study, the PFAS during late pregnancy to PFAS in newborns at delivery could still have weeks of exposure not accounted for, so such variability would bias the present results toward the null. Last, the findings derived from the African American pregnant cohort, specifically located within the Atlanta area, could limit the generalizability of the present study to a broader population across varying geographic areas. However, we also acknowledge that this is a strength of our study given that no prior work on prenatal PFAS exposures has focused on exclusively African American women, who are underrepresented in epidemiological research.
In conclusion, our results show that, in this study population, PFAS exposures were ubiquitous among pregnant African American mothers and their newborns, with PFHxS being the most predominant compound. Furthermore, the PFAS concentrations exhibited notable variability and differing transfer efficiency among individuals over pregnancy. Delineating patterns of PFAS exposure and identifying important predictors can contribute to our understanding of health burden within this underrepresented population, informing more equitable public health strategies, including developing targeted interventions on potential exposure routes and improving maternal and neonatal health.
Community involvement has been integral to this study from its inception. Focus groups and a community advisory board played a critical role in shaping the study’s focus on specific exposures, including environmental toxicants and psychosocial stressors.37,38 Black Women Wellness further contributed to the study design, particularly given the involvement of Black women clinical research coordinators in recruitment and study visits. Ongoing efforts by the study team include engaging participants to identify the best methods for reporting chemical exposure levels, with continuous feedback sought from both the community and participants. Thus, the study’s design and focus were shaped through extensive community engagement and collaboration, ensuring that the research remains relevant to the specific needs and experiences of US-born African Americans, with particular attention to the most impactful social, biological, and environmental exposures.
Future research directions should focus on more time points for PFAS measurement during and post pregnancy among pregnant people and their children, capturing a more comprehensive longitudinal depiction on PFAS levels over pregnancy and postnatal periods. In addition, future research should incorporate a broader spectrum of predictors and physiological factors that may affect PFAS levels and how changes in PFAS levels can have long-term health impacts on children exposed to PFAS in utero. Building upon the present study’s findings, toxicological studies examining the effect of PFAS exposure during critical windows of susceptibility on maternal and child health should be considered in both in vivo and in vitro settings. Clarifying the toxicological mechanisms of PFAS could provide a crucial basis for developing public health strategies to protect vulnerable and minor populations.
Supplementary Material
Acknowledgments
We thank all the members from the Environmental Metabolomics and Exposomics Research Group at Emory (EMERGE) for their valuable input and feedback on this project. In addition, we are grateful for our colleagues—Nathan Mutic, Cierra Johnson, Erin Williams, Estefani Ignacio Gallegos, Nikolay Patrushev, Kristi Maxwell Logue, Castalia Thorne, Shirleta Reid, and Cassandra Hall—and the clinical health care practitioners and staff at the prenatal recruiting sites for helping with data and sample collection and logistics and sample chemical analyses in the laboratories.
Research reported in this publication was supported by the Environmental Influences on Child Health Outcomes (ECHO) program, Office of the Director, National Institutes of Health, under award nos. 5U2COD023375-05/A03-3824 and UG3/UH3OD023318; the National Institutes of Health (NIH) research grants (R21ES032117, R01ES035738, R01NR014800, R01MD009064, R24ES029490, R01MD009746); NIH center grants (P50ES02607, P30ES019776, UH3OD023318, U2CES026560, U2CES026542); the National Institute of Environmental Health under award no. 5T32ES12870; and the US Environmental Protection Agency center grant 83615301. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conclusions and opinions are those of the individual authors and do not necessarily reflect the policies or views of EHP Publishing or the National Institute of Environmental Health Sciences.
References
- 1.Paul AG, Jones KC, Sweetman AJ. 2009. A first global production, emission, and environmental inventory for perfluorooctane sulfonate. Environ Sci Technol 43(2):386–392, PMID: 19238969, 10.1021/es802216n. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Z, Sarkar D, Biswas JK, Datta R. 2022. Biodegradation of per- and polyfluoroalkyl substances (PFAS): a review. Bioresour Technol 344(pt B):126223, PMID: 34756980, 10.1016/j.biortech.2021.126223. [DOI] [PubMed] [Google Scholar]
- 3.Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. 2019. A review of the pathways of human exposure to poly-and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol 29(2):131–147, PMID: 30470793, 10.1038/s41370-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ATSDR (Agency for Toxic Substances and Disease Registry). 2022. Per- and Polyfluoroalkyl Substances (PFAS) and Your Health. What are the health effects of PFAS? https://www.atsdr.cdc.gov/pfas/health-effects/index.html [accessed 30 October 2024].
- 5.Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. 2007. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci 99(2):366–394, PMID: 17519394, 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- 6.Fenton SE, Ducatman A, Boobis A, DeWitt JC, Lau C, Ng C, et al. . 2021. Per- and polyfluoroalkyl substance toxicity and human health review: current state of knowledge and strategies for informing future research. Environ Toxicol Chem 40(3):606–630, PMID: 33017053, 10.1002/etc.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US EPA (US Environmental Protection Agency). 2023. Wolverine World Wide Tannery. EPA and EGLE Working on Cleanup of Contamination. Updated 21 February 2023. https://www.epa.gov/mi/wolverine-world-wide-tannery [accessed 30 October 2024].
- 8.Calafat AM, Wong L-Y, Kuklenyik Z, Reidy JA, Needham LL. 2007. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ Health Perspect 115(11):1596–1602, PMID: 18007991, 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brase RA, Mullin EJ, Spink DC. 2021. Legacy and emerging per- and polyfluoroalkyl substances: analytical techniques, environmental fate, and health effects. Int J Mol Sci 22(3):995, PMID: 33498193, 10.3390/ijms22030995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giesy JP, Kannan K. 2002. Perfluorochemical surfactants in the environment. Environ Sci Technol 36(7):146A–152A, PMID: 11999053, 10.1021/es022253t. [DOI] [PubMed] [Google Scholar]
- 11.Andrews DQ, Naidenko OV. 2020. Population-wide exposure to per- and polyfluoroalkyl substances from drinking water in the United States. Environ Sci Technol Lett 7(12):931–936, 10.1021/acs.estlett.0c00713. [DOI] [Google Scholar]
- 12.Morello-Frosch R, Cushing LJ, Jesdale BM, Schwartz JM, Guo W, Guo T, et al. . 2016. Environmental chemicals in an urban population of pregnant women and their newborns from San Francisco. Environ Sci Technol 50(22):12464–12472, PMID: 27700069, 10.1021/acs.est.6b03492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang H, Kim H-S, Yoon YS, Lee J, Kho Y, Lee J, et al. . 2021. Placental transfer and composition of perfluoroalkyl substances (PFASs): a Korean birth panel of parent-infant triads. Toxics 9(7):168, PMID: 34357911, 10.3390/toxics9070168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beesoon S, Webster GM, Shoeib M, Harner T, Benskin JP, Martin JW. 2011. Isomer profiles of perfluorochemicals in matched maternal, cord, and house dust samples: manufacturing sources and transplacental transfer. Environ Health Perspect 119(11):1659–1664, PMID: 21757419, 10.1289/ehp.1003265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao X, Ni W, Zhu S, Wu Y, Cui Y, Ma J, et al. . 2021. Per-and polyfluoroalkyl substances exposure during pregnancy and adverse pregnancy and birth outcomes: a systematic review and meta-analysis. Environ Res 201:111632, PMID: 34237336, 10.1016/j.envres.2021.111632. [DOI] [PubMed] [Google Scholar]
- 16.Fei C, McLaughlin JK, Tarone RE, Olsen J. 2007. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ Health Perspect 115(11):1677–1682, PMID: 18008003, 10.1289/ehp.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Souza MCO, Saraiva MCP, Honda M, Barbieri MA, Bettiol H, Barbosa F, et al. . 2020. Exposure to per-and polyfluorinated alkyl substances in pregnant Brazilian women and its association with fetal growth. Environ Res 187:109585, PMID: 32442788, 10.1016/j.envres.2020.109585. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman K, Webster TF, Weisskopf MG, Weinberg J, Vieira VM. 2010. Exposure to polyfluoroalkyl chemicals and attention deficit/hyperactivity disorder in U.S. children 12–15 years of age. Environ Health Perspect 118(12):1762–1767, PMID: 20551004, 10.1289/ehp.1001898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savitz DA, Stein CR, Bartell SM, Elston B, Gong J, Shin H-M, et al. . 2012. Perfluorooctanoic acid exposure and pregnancy outcome in a highly exposed community. Epidemiology 23(3):386–392, PMID: 22370857, 10.1097/EDE.0b013e31824cb93b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L, Tong C, Huo X, Zhang J, Tian Y, Shanghai Birth Cohort. 2021. Prenatal exposure to perfluoroalkyl and polyfluoroalkyl substances and birth outcomes: a longitudinal cohort with repeated measurements. Chemosphere 267:128899, PMID: 33220988, 10.1016/j.chemosphere.2020.128899. [DOI] [PubMed] [Google Scholar]
- 21.Oh J, Bennett DH, Tancredi DJ, Calafat AM, Schmidt RJ, Hertz-Picciotto I, et al. . 2022. Longitudinal changes in maternal serum concentrations of per- and polyfluoroalkyl substances from pregnancy to two years postpartum. Environ Sci Technol 56(16):11449–11459, PMID: 35904360, 10.1021/acs.est.1c07970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kingsley SL, Eliot MN, Kelsey KT, Calafat AM, Ehrlich S, Lanphear BP, et al. . 2018. Variability and predictors of serum perfluoroalkyl substance concentrations during pregnancy and early childhood. Environ Res 165:247–257, PMID: 29734025, 10.1016/j.envres.2018.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fromme H, Mosch C, Morovitz M, Alba-Alejandre I, Boehmer S, Kiranoglu M, et al. . 2010. Pre- and postnatal exposure to perfluorinated compounds (PFCs). Environ Sci Technol 44(18):7123–7129, PMID: 20722423, 10.1021/es101184f. [DOI] [PubMed] [Google Scholar]
- 24.Kato S, Itoh S, Yuasa M, Baba T, Miyashita C, Sasaki S, et al. . 2016. Association of perfluorinated chemical exposure in utero with maternal and infant thyroid hormone levels in the Sapporo cohort of Hokkaido Study on the Environment and Children’s Health. Environ Health Prev Med 21(5):334–344, PMID: 27137816, 10.1007/s12199-016-0534-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang M, Yu CH, Wang G, Buckley JP, Hong X, Pearson C, et al. . 2022. Longitudinal trajectories and determinants of plasma per- and polyfluoroalkyl substance (PFAS) levels from birth to early childhood and metabolomic associations: a pilot study in the Boston Birth Cohort. Precis Nutr 1(1):e00004, PMID: 36936201. [PMC free article] [PubMed] [Google Scholar]
- 26.Blomberg AJ, Shih Y-H, Messerlian C, Jørgensen LH, Weihe P, Grandjean P. 2021. Early-life associations between per-and polyfluoroalkyl substances and serum lipids in a longitudinal birth cohort. Environ Res 200:111400, PMID: 34081971, 10.1016/j.envres.2021.111400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McAdam J, Bell EM. 2023. Determinants of maternal and neonatal PFAS concentrations: a review. Environ Health 22(1):41, PMID: 37161484, 10.1186/s12940-023-00992-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richterová D, Fábelová L, Patayová H, Pulkrabová J, Lanková D, Rausová K, et al. . 2018. Determinants of prenatal exposure to perfluoroalkyl substances in the Slovak birth cohort. Environ Int 121(pt 2):1304–1310, PMID: 30420127, 10.1016/j.envint.2018.10.051. [DOI] [PubMed] [Google Scholar]
- 29.Chang C-J, Ryan PB, Smarr MM, Kannan K, Panuwet P, Dunlop AL, et al. . 2021. Serum per-and polyfluoroalkyl substance (PFAS) concentrations and predictors of exposure among pregnant African American women in the Atlanta area, Georgia. Environ Res 198:110445, PMID: 33186575, 10.1016/j.envres.2020.110445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sagiv SK, Rifas-Shiman SL, Webster TF, Mora AM, Harris MH, Calafat AM, et al. . 2015. Sociodemographic and perinatal predictors of early pregnancy per- and polyfluoroalkyl substance (PFAS) concentrations. Environ Sci Technol 49(19):11849–11858, PMID: 26333069, 10.1021/acs.est.5b02489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halldorsson TI, Fei C, Olsen J, Lipworth L, McLaughlin JK, Olsen SF. 2008. Dietary predictors of perfluorinated chemicals: a study from the Danish National Birth Cohort. Environ Sci Technol 42(23):8971–8977, PMID: 19192827, 10.1021/es801907r. [DOI] [PubMed] [Google Scholar]
- 32.Braveman P, Dominguez TP, Burke W, Dolan SM, Stevenson DK, Jackson FM, et al. . 2021. Explaining the Black-White disparity in preterm birth: a consensus statement from a multi-disciplinary scientific work group convened by the March of Dimes. Front Reprod Health 3:684207, PMID: 36303973, 10.3389/frph.2021.684207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosen Vollmar AK, Lin EZ, Nason SL, Santiago K, Johnson CH, Ma X, et al. . 2023. Per- and polyfluoroalkyl substances (PFAS) and thyroid hormone measurements in dried blood spots and neonatal characteristics: a pilot study. J Expo Sci Environ Epidemiol 33(5):737–747, PMID: 37730931, 10.1038/s41370-023-00603-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spliethoff HM, Tao L, Shaver SM, Aldous KM, Pass KA, Kannan K, et al. . 2008. Use of newborn screening program blood spots for exposure assessment: declining levels of perfluorinated compounds in New York State infants. Environ Sci Technol 42(14):5361–5367, PMID: 18754394, 10.1021/es8006244. [DOI] [PubMed] [Google Scholar]
- 35.Lin EZ, Nason SL, Zhong A, Fortner J, Godri Pollitt KJ. 2023. Trace analysis of per- and polyfluorinated alkyl substances (PFAS) in dried blood spots – demonstration of reproducibility and comparability to venous blood samples. Sci Total Environ 883:163530, PMID: 37094673, 10.1016/j.scitotenv.2023.163530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kato K, Wanigatunga AA, Needham LL, Calafat AM. 2009. Analysis of blood spots for polyfluoroalkyl chemicals. Anal Chim Acta 656(1–2):51–55, PMID: 19932814, 10.1016/j.aca.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Corwin EJ, Hogue CJ, Pearce B, Hill CC, Read TD, Mulle J, et al. . 2017. Protocol for the Emory University African American Vaginal, Oral, and Gut Microbiome in Pregnancy cohort study. [Published correction appears in BMC Pregnancy Childbirth 17(1):161.] BMC Pregnancy Childbirth 17(1):395, PMID: 28571577, 10.1186/s12884-017-1550-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brennan PA, Dunlop AL, Smith AK, Kramer M, Mulle J, Corwin EJ. 2019. Protocol for the Emory University African American Maternal Stress and Infant Gut Microbiome cohort study. BMC Pediatr 19(1):246, PMID: 31331308, 10.1186/s12887-019-1630-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Green NS, Damus K, Simpson JL, Iams J, Reece EA, Hobel CJ, et al. . 2005. Research agenda for preterm birth: recommendations from the March of Dimes. Am J Obstet Gynecol 193(3 pt 1):626–635, PMID: 16150253, 10.1016/j.ajog.2005.02.106. [DOI] [PubMed] [Google Scholar]
- 40.Taibl KR, Dunlop AL, Barr DB, Li Y-Y, Eick SM, Kannan K, et al. . 2023. Newborn metabolomic signatures of maternal per-and polyfluoroalkyl substance exposure and reduced length of gestation. Nat Commun 14(1):3120, PMID: 37253729, 10.1038/s41467-023-38710-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Georgia Department of Public Health. 2024. Newborn Screening: Women and Children. NBS Providers. https://dph.georgia.gov/NBS/nbs-policies-and-procedures [accessed 30 October 2024].
- 42.Kannan K, Stathis A, Mazzella MJ, Andra SS, Barr DB, Hecht SS, et al. . 2021. Quality assurance and harmonization for targeted biomonitoring measurements of environmental organic chemicals across the Children’s Health Exposure Analysis Resource laboratory network. Int J Hyg Environ Health 234:113741, PMID: 33773388, 10.1016/j.ijheh.2021.113741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Honda M, Robinson M, Kannan K. 2018. A rapid method for the analysis of perfluorinated alkyl substances in serum by hybrid solid-phase extraction. Environ Chem 15(2):92–99, 10.1071/EN17192. [DOI] [Google Scholar]
- 44.Ma W, Kannan K, Wu Q, Bell EM, Druschel CM, Caggana M, et al. . 2013. Analysis of polyfluoroalkyl substances and bisphenol A in dried blood spots by liquid chromatography tandem mass spectrometry. Anal Bioanal Chem 405(12):4127–4138, PMID: 23404131, 10.1007/s00216-013-6787-3. [DOI] [PubMed] [Google Scholar]
- 45.Adam BW, Alexander JR, Smith SJ, Chace DH, Loeber JG, Elvers LH, et al. . 2000. Recoveries of phenylalanine from two sets of dried-blood-spot reference materials: prediction from hematocrit, spot volume, and paper matrix. Clin Chem 46(1):126–128, PMID: 10620584, 10.1093/clinchem/46.1.126. [DOI] [PubMed] [Google Scholar]
- 46.Hornung RW, Reed LD. 1990. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg 5(1):46–51, 10.1080/1047322X.1990.10389587. [DOI] [Google Scholar]
- 47.Ehresman DJ, Froehlich JW, Olsen GW, Chang S-C, Butenhoff JL. 2007. Comparison of human whole blood, plasma, and serum matrices for the determination of perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and other fluorochemicals. Environ Res 103(2):176–184, PMID: 16893538, 10.1016/j.envres.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 48.Poothong S, Papadopoulou E, Lundanes E, Padilla-Sánchez JA, Thomsen C, Haug LS. 2019. Dried blood spots for reliable biomonitoring of poly-and perfluoroalkyl substances (PFASs). Sci Total Environ 655:1420–1426, PMID: 30577133, 10.1016/j.scitotenv.2018.11.214. [DOI] [PubMed] [Google Scholar]
- 49.Goin DE, Izano MA, Eick SM, Padula AM, DeMicco E, Woodruff TJ, et al. . 2021. Maternal experience of multiple hardships and fetal growth: extending environmental mixtures methodology to social exposures. Epidemiology 32(1):18–26, PMID: 33031217, 10.1097/EDE.0000000000001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeLuca NM, Thomas K, Mullikin A, Slover R, Stanek LW, Pilant AN, et al. . 2023. Geographic and demographic variability in serum PFAS concentrations for pregnant women in the United States. J Expo Sci Environ Epidemiol 33(5):710–724, PMID: 36697764, 10.1038/s41370-023-00520-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gardener H, Sun Q, Grandjean P. 2021. PFAS concentration during pregnancy in relation to cardiometabolic health and birth outcomes. Environ Res 192:110287, PMID: 33038367, 10.1016/j.envres.2020.110287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boronow KE, Brody JG, Schaider LA, Peaslee GF, Havas L, Cohn BA. 2019. Serum concentrations of PFASs and exposure-related behaviors in African American and non-Hispanic White women. J Expo Sci Environ Epidemiol 29(2):206–217, PMID: 30622332, 10.1038/s41370-018-0109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Romano ME, Xu Y, Calafat AM, Yolton K, Chen A, Webster GM, et al. . 2016. Maternal serum perfluoroalkyl substances during pregnancy and duration of breastfeeding. Environ Res 149:239–246, PMID: 27179585, 10.1016/j.envres.2016.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.CDC (Centers for Disease Control and Prevention). 2022. National Report on Human Exposure to Environmental Chemicals. Updated 24 March 2022. https://www.cdc.gov/environmental-exposure-report/data-tables/index.html [accessed 30 October 2024].
- 55.DeLuca NM, Minucci JM, Mullikin A, Slover R, Cohen Hubal EA. 2022. Human exposure pathways to poly- and perfluoroalkyl substances (PFAS) from indoor media: a systematic review. Environ Int 162:107149, PMID: 35240384, 10.1016/j.envint.2022.107149. [DOI] [PubMed] [Google Scholar]
- 56.Hu XC, Dassuncao C, Zhang X, Grandjean P, Weihe P, Webster GM, et al. . 2018. Can profiles of poly- and perfluoroalkyl substances (PFASs) in human serum provide information on major exposure sources? Environ Health 17(1):11, PMID: 29391068, 10.1186/s12940-018-0355-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goosey E, Harrad S. 2011. Perfluoroalkyl compounds in dust from Asian, Australian, European, and North American homes and UK cars, classrooms, and offices. Environ Int 37(1):86–92, PMID: 20810169, 10.1016/j.envint.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 58.Strynar MJ, Lindstrom AB. 2008. Perfluorinated compounds in house dust from Ohio and North Carolina, USA. Environ Sci Technol 42(10):3751–3756, PMID: 18546718, 10.1021/es7032058. [DOI] [PubMed] [Google Scholar]
- 59.Jia J, Duan L, Dong B, Dong Q, Liu Y, Yu W, et al. . 2023. Perfluoroalkyl and polyfluoroalkyl substances in cord serum of newborns and their potential factors. Chemosphere 313:137525, PMID: 36521747, 10.1016/j.chemosphere.2022.137525. [DOI] [PubMed] [Google Scholar]
- 60.Fábelová L, Beneito A, Casas M, Colles A, Dalsager L, Den Hond E, et al. . 2023. PFAS levels and exposure determinants in sensitive population groups. Chemosphere 313:137530, PMID: 36509187, 10.1016/j.chemosphere.2022.137530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guo J, Zhang J, Wang Z, Zhang L, Qi X, Zhang Y, et al. . 2021. Umbilical cord serum perfluoroalkyl substance mixtures in relation to thyroid function of newborns: findings from Sheyang Mini Birth Cohort Study. Chemosphere 273:129664, PMID: 33493812, 10.1016/j.chemosphere.2021.129664. [DOI] [PubMed] [Google Scholar]
- 62.Ghassabian A, Bell EM, Ma W-L, Sundaram R, Kannan K, Buck Louis GM, et al. . 2018. Concentrations of perfluoroalkyl substances and bisphenol A in newborn dried blood spots and the association with child behavior. Environ Pollut 243(pt B):1629–1636, PMID: 30296759, 10.1016/j.envpol.2018.09.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buck Louis GM, Yeung E, Kannan K, Maisog J, Zhang C, Grantz KL, et al. . 2019. Patterns and variability of endocrine-disrupting chemicals during pregnancy: implications for understanding the exposome of normal pregnancy. Epidemiology 30(suppl 2):S65–S75, PMID: 31569155, 10.1097/EDE.0000000000001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Glynn A, Berger U, Bignert A, Ullah S, Aune M, Lignell S, et al. . 2012. Perfluorinated alkyl acids in blood serum from primiparous women in Sweden: serial sampling during pregnancy and nursing, and temporal trends 1996–2010. Environ Sci Technol 46(16):9071–9079, PMID: 22770559, 10.1021/es301168c. [DOI] [PubMed] [Google Scholar]
- 65.Hu Y, Han F, Wang Y, Zhong Y, Zhan J, Liu J. 2023. Trimester-specific hemodynamics of per-and polyfluoroalkyl substances and its relation to lipid profile in pregnant women. J Hazard Mater 460:132339, PMID: 37660622, 10.1016/j.jhazmat.2023.132339. [DOI] [PubMed] [Google Scholar]
- 66.Kato K, Wong L-Y, Chen A, Dunbar C, Webster GM, Lanphear BP, et al. . 2014. Changes in serum concentrations of maternal poly- and perfluoroalkyl substances over the course of pregnancy and predictors of exposure in a multiethnic cohort of Cincinnati, Ohio pregnant women during 2003–2006. Environ Sci Technol 48(16):9600–9608, PMID: 25026485, 10.1021/es501811k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. . 2007. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115(9):1298–1305, PMID: 17805419, 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Y, Fletcher T, Mucs D, Scott K, Lindh CH, Tallving P, et al. . 2018. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup Environ Med 75(1):46–51, PMID: 29133598, 10.1136/oemed-2017-104651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Worley RR, Moore SM, Tierney BC, Ye X, Calafat AM, Campbell S, et al. . 2017. Per- and polyfluoroalkyl substances in human serum and urine samples from a residentially exposed community. Environ Int 106:135–143, PMID: 28645013, 10.1016/j.envint.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salas SP, Marshall G, Gutiérrez BL, Rosso P. 2006. Time course of maternal plasma volume and hormonal changes in women with preeclampsia or fetal growth restriction. Hypertension 47(2):203–208, PMID: 16380519, 10.1161/01.HYP.0000200042.64517.19. [DOI] [PubMed] [Google Scholar]
- 71.Pan Y, Zhu Y, Zheng T, Cui Q, Buka SL, Zhang B, et al. . 2017. Novel chlorinated polyfluorinated ether sulfonates and legacy per-/polyfluoroalkyl substances: placental transfer and relationship with serum albumin and glomerular filtration rate. Environ Sci Technol 51(1):634–644, PMID: 27931097, 10.1021/acs.est.6b04590. [DOI] [PubMed] [Google Scholar]
- 72.Taibl KR, Liang D, Dunlop AL, Barr DB, Smith MR, Steenland K, et al. . 2023. Pregnancy-related hemodynamic biomarkers in relation to trimester-specific maternal per-and polyfluoroalkyl substances exposures and adverse birth outcomes. Environ Pollut 323:121331, PMID: 36813097, 10.1016/j.envpol.2023.121331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Manzano-Salgado CB, Casas M, Lopez-Espinosa M-J, Ballester F, Basterrechea M, Grimalt JO, et al. . 2015. Transfer of perfluoroalkyl substances from mother to fetus in a Spanish birth cohort. Environ Res 142:471–478, PMID: 26257032, 10.1016/j.envres.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 74.Manzano-Salgado CB, Casas M, Lopez-Espinosa M-J, Ballester F, Martinez D, Ibarluzea J, et al. . 2016. Variability of perfluoroalkyl substance concentrations in pregnant women by socio-demographic and dietary factors in a Spanish birth cohort. Environ Int 92–93:357–365, PMID: 27132161, 10.1016/j.envint.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 75.Papadopoulou E, Sabaredzovic A, Namork E, Nygaard UC, Granum B, Haug LS. 2016. Exposure of Norwegian toddlers to perfluoroalkyl substances (PFAS): the association with breastfeeding and maternal PFAS concentrations. Environ Int 94:687–694, PMID: 27453094, 10.1016/j.envint.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 76.Cariou R, Veyrand B, Yamada A, Berrebi A, Zalko D, Durand S, et al. . 2015. Perfluoroalkyl acid (PFAA) levels and profiles in breast milk, maternal and cord serum of French women and their newborns. Environ Int 84:71–81, PMID: 26232143, 10.1016/j.envint.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 77.Cai D, Li Q-Q, Chu C, Wang S-Z, Tang Y-T, Appleton AA, et al. . 2020. High trans-placental transfer of perfluoroalkyl substances alternatives in the matched maternal-cord blood serum: evidence from a birth cohort study. Sci Total Environ 705:135885, PMID: 31841927, 10.1016/j.scitotenv.2019.135885. [DOI] [PubMed] [Google Scholar]
- 78.Gützkow KB, Haug LS, Thomsen C, Sabaredzovic A, Becher G, Brunborg G. 2012. Placental transfer of perfluorinated compounds is selective—a Norwegian Mother and Child sub-cohort study. Int J Hyg Environ Health 215(2):216–219, PMID: 21937271, 10.1016/j.ijheh.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 79.Liew Z, Goudarzi H, Oulhote Y. 2018. Developmental exposures to perfluoroalkyl substances (PFASs): an update of associated health outcomes. Curr Environ Health Rep 5(1):1–19, PMID: 29556975, 10.1007/s40572-018-0173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim S, Choi K, Ji K, Seo J, Kho Y, Park J, et al. . 2011. Trans-placental transfer of thirteen perfluorinated compounds and relations with fetal thyroid hormones. Environ Sci Technol 45(17):7465–7472, PMID: 21805959, 10.1021/es202408a. [DOI] [PubMed] [Google Scholar]
- 81.Kim S-K, Lee KT, Kang CS, Tao L, Kannan K, Kim K-R, et al. . 2011. Distribution of perfluorochemicals between sera and milk from the same mothers and implications for prenatal and postnatal exposures. Environ Pollut 159(1):169–174, PMID: 20932617, 10.1016/j.envpol.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 82.Abrahamsson D, Siddharth A, Robinson JF, Soshilov A, Elmore S, Cogliano V, et al. . 2022. Modeling the transplacental transfer of small molecules using machine learning: a case study on per- and polyfluorinated substances (PFAS). J Expo Sci Environ Epidemiol 32(6):808–819, PMID: 36207486, 10.1038/s41370-022-00481-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang T, Sun H, Lin Y, Qin X, Zhang Y, Geng X, et al. . 2013. Distribution of poly-and perfluoroalkyl substances in matched samples from pregnant women and carbon chain length related maternal transfer. Environ Sci Technol 47(14):7974–7981, PMID: 23777259, 10.1021/es400937y. [DOI] [PubMed] [Google Scholar]
- 84.Nelson JW, Edhlund BL, Johnson J, Rosebush CE, Holmquist ZS, Swan SH, et al. . 2016. Assessing a new method for measuring fetal exposure to mercury: newborn bloodspots. Int J Environ Res Public Health 13(7):692, PMID: 27409626, 10.3390/ijerph13070692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang J, Pearl M, Jacob P III, DeLorenze GN, Benowitz NL, Yu L, et al. . 2013. Levels of cotinine in dried blood specimens from newborns as a biomarker of maternal smoking close to the time of delivery. Am J Epidemiol 178(11):1648–1654, PMID: 24068198, 10.1093/aje/kwt182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Appel M, Forsthuber M, Ramos R, Widhalm R, Granitzer S, Uhl M, et al. . 2022. The transplacental transfer efficiency of per- and polyfluoroalkyl substances (PFAS): a first meta-analysis. J Toxicol Environ Health B Crit Rev 25(1):23–42, PMID: 34930098, 10.1080/10937404.2021.2009946. [DOI] [PubMed] [Google Scholar]
- 87.Hanssen L, Dudarev AA, Huber S, Odland JØ, Nieboer E, Sandanger TM. 2013. Partition of perfluoroalkyl substances (PFASs) in whole blood and plasma, assessed in maternal and umbilical cord samples from inhabitants of Arctic Russia and Uzbekistan. Sci Total Environ 447:430–437, PMID: 23410865, 10.1016/j.scitotenv.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 88.Jian J-M, Chen D, Han F-J, Guo Y, Zeng L, Lu X, et al. . 2018. A short review on human exposure to and tissue distribution of per- and polyfluoroalkyl substances (PFASs). Sci Total Environ 636:1058–1069, PMID: 29913568, 10.1016/j.scitotenv.2018.04.380. [DOI] [PubMed] [Google Scholar]
- 89.Brantsæter AL, Whitworth KW, Ydersbond TA, Haug LS, Haugen M, Knutsen HK, et al. . 2013. Determinants of plasma concentrations of perfluoroalkyl substances in pregnant Norwegian women. Environ Int 54:74–84, PMID: 23419425, 10.1016/j.envint.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rylander C, Sandanger TM, Frøyland L, Lund E. 2010. Dietary patterns and plasma concentrations of perfluorinated compounds in 315 Norwegian women: the NOWAC postgenome study. Environ Sci Technol 44(13):5225–5232, PMID: 20527765, 10.1021/es100224q. [DOI] [PubMed] [Google Scholar]
- 91.Inoue K, Okada F, Ito R, Kato S, Sasaki S, Nakajima S, et al. . 2004. Perfluorooctane sulfonate (PFOS) and related perfluorinated compounds in human maternal and cord blood samples: assessment of PFOS exposure in a susceptible population during pregnancy. Environ Health Perspect 112(11):1204–1207, PMID: 15289168, 10.1289/ehp.6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ali SM, Lindström M. 2006. Socioeconomic, psychosocial, behavioural, and psychological determinants of BMI among young women: differing patterns for underweight and overweight/obesity. Eur J Public Health 16(3):325–331, PMID: 16162598, 10.1093/eurpub/cki187. [DOI] [PubMed] [Google Scholar]
- 93.Kannan K, Corsolini S, Falandysz J, Fillmann G, Kumar KS, Loganathan BG, et al. . 2004. Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ Sci Technol 38(17):4489–4495, PMID: 15461154, 10.1021/es0493446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.