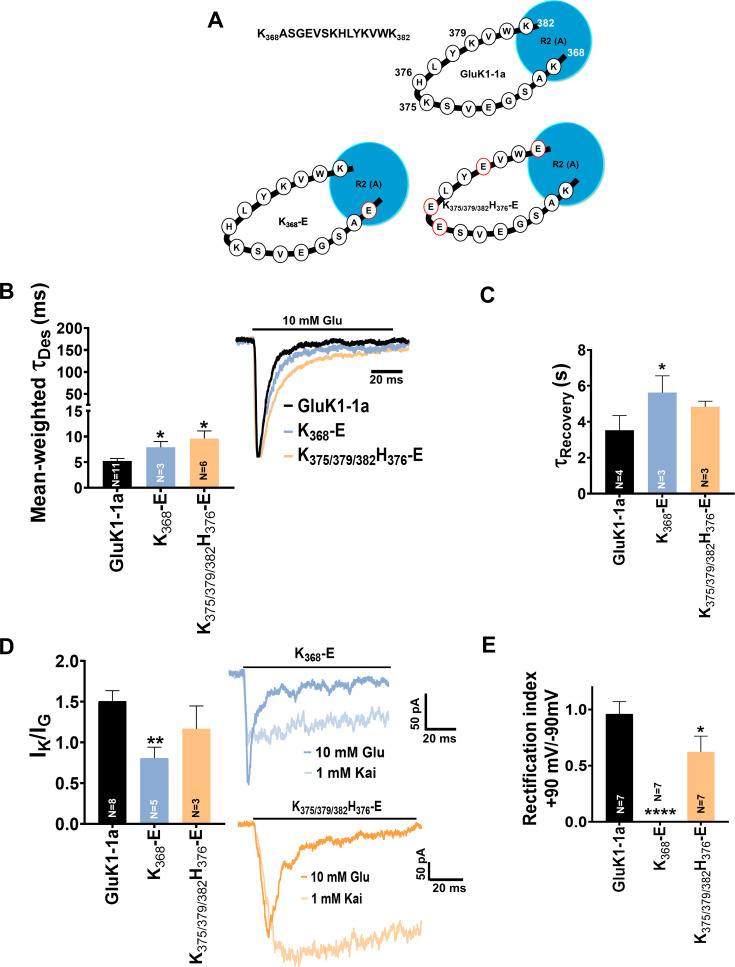
Figure 4. Mutation of GluK1-1a splice insert residues affects the desensitization and recovery kinetics of the receptor.
Bar graphs (mean ± SEM) show a comparison between wild-type and mutant receptors for different kinetic properties. (A) Schematic representation of 15 residues amino-terminal domain (ATD) splice (K368ASGEVSKHLYKVWK382) in wild-type and mutant receptors under study (B) Mean-weighted Tau (τDes) values for GluK1-1a wild-type and mutant receptors in the presence of 10 mM glutamate. (C) Tau (τRecovery) recovery values for GluK1-1a and mutants. (D) The ratio of the peak amplitudes evoked in the presence of 1 mM kainate and 10 mM glutamate is shown for GluK1-1a mutants.(E) The rectification index represented by the ratio of currents evoked by 10 mM glutamate application at +90 mV and –90 mV for the wild-type and mutant receptors is shown. The wild-type GluK1 splice variant data is the same as from Figure 2A and is replotted here for comparison. Error bars indicate mean ± SEM, N in each bar represents the number of cells used for analysis, and * indicates the significance at a 95% confidence interval.