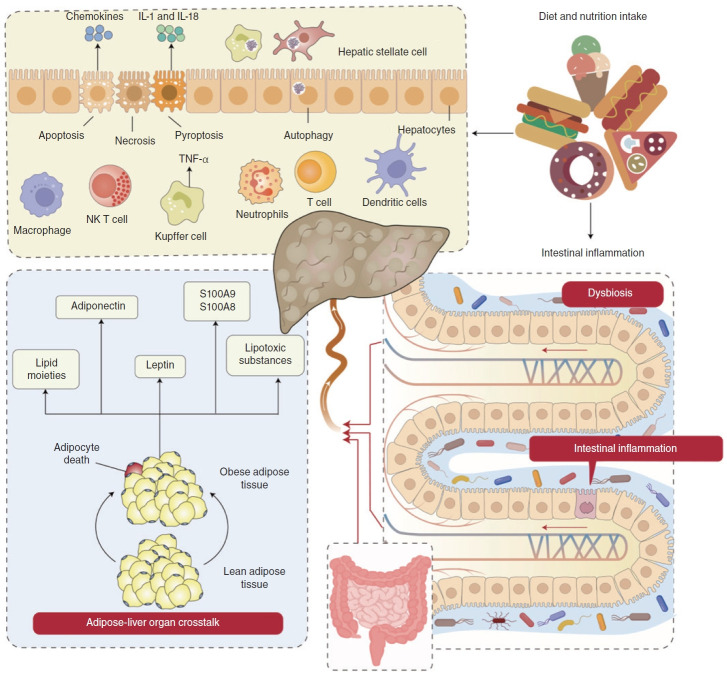
Figure 2.
Immune dysregulation in MASLD through the interaction of the gut, liver, and adipose organs. The immune dysregulation in MASLD involves hepatocyte death, the adipocyte-liver axis and gut dysbiosis. (1) High fat diets (HFD) consumption leads to gut barrier dysfunction, escalating intestinal inflammation and triggering an ectopic immune response. Damage to the intestinal barrier facilitates the passage of bacteria or bacterial components into the bloodstream, essential for hepatocyte death and MASLD progression [12]. (2) HFD consumption transforms lean adipose tissue into obese adipose tissue. Obese adipose tissue releases adiponectin, leptin and lipid moieties like palmitic acids, ceramide, IL-6 and TNF, inducing cell stress and hepatocyte death in MASLD.83,84 (3) Both gut dysbiosis and obese adipose tissue lead to hepatocyte death, which mainly encompasses apoptosis, necroptosis and pyroptosis. These factors activate KCs, producing TNF, TRAIL and FAS ligands by engulfing apoptotic bodies, thereby stimulating the secretion of chemokines and triggering hepatocyte apoptosis [96]. These factors further damage hepatocytes, leading to necroptosis and pyroptosis. This process involves the release of IL-1 and IL-18 into the bloodstream, influencing autophagy alterations in hepatocytes and nonparenchymal cells like KCs and HSCs [103]. All these factors then activate the mucosal immune cells such as macrophages, NK T cells, Kupffer cells, neutrophils, T cells and DCs to release inflammatory cytokines and chemokines, further leading to hepatocyte death. MASLD, metabolic dysfunctionassociated steatotic liver disease; IL, interleukin; KCs, Kupffer cells; TRAIL, tumour necrosis factor-related apoptosis-inducing ligand; HSCs, hepatic stellate cells; DCs, dendritic cells.