Abstract
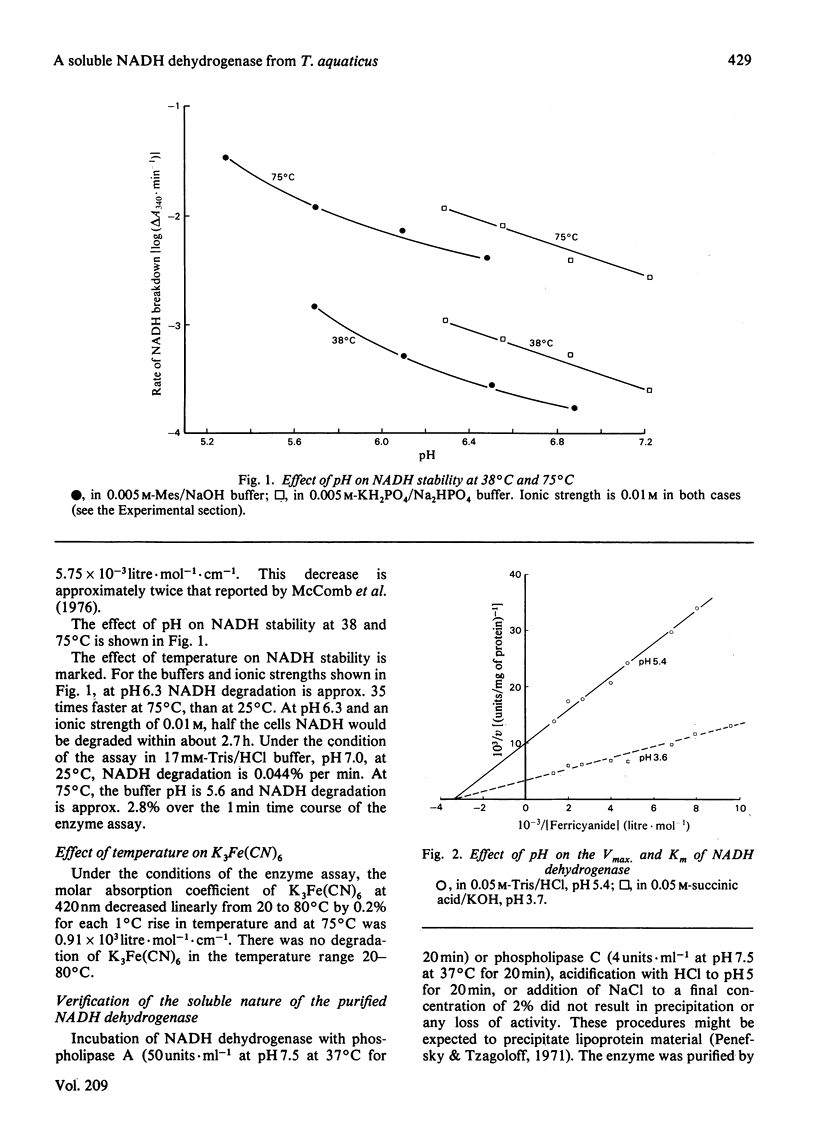
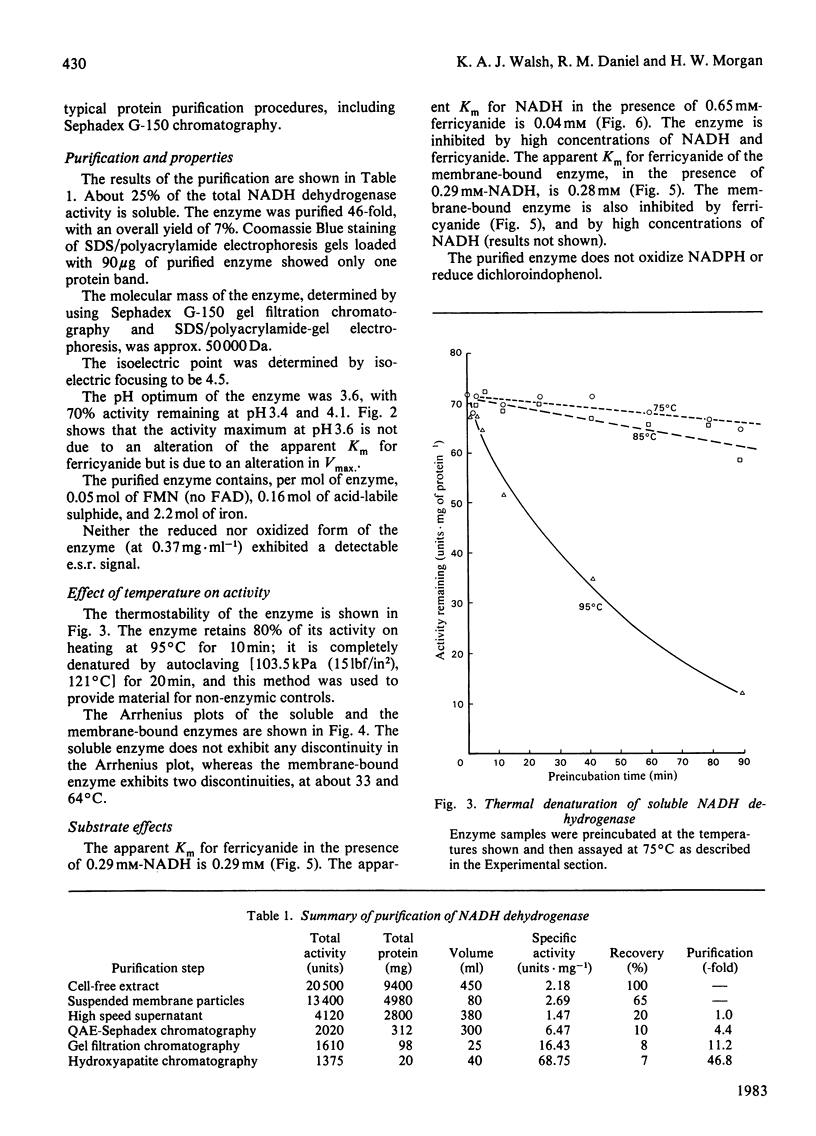
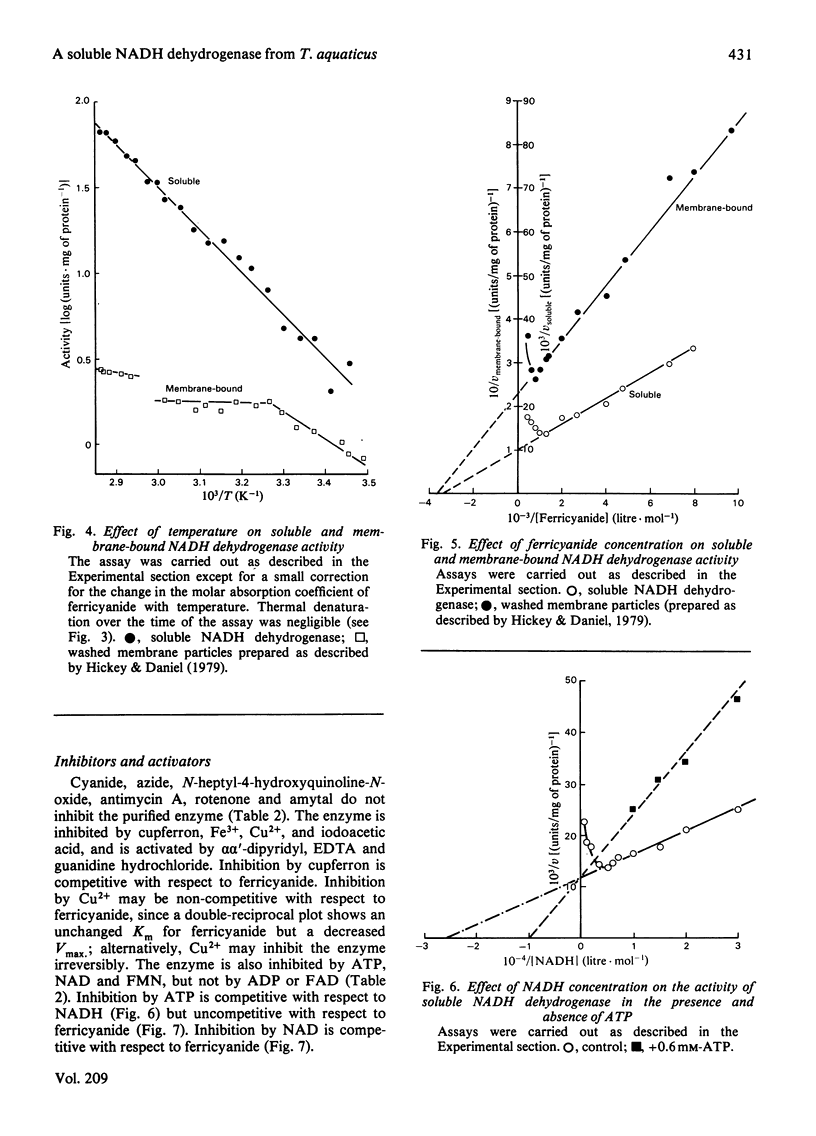
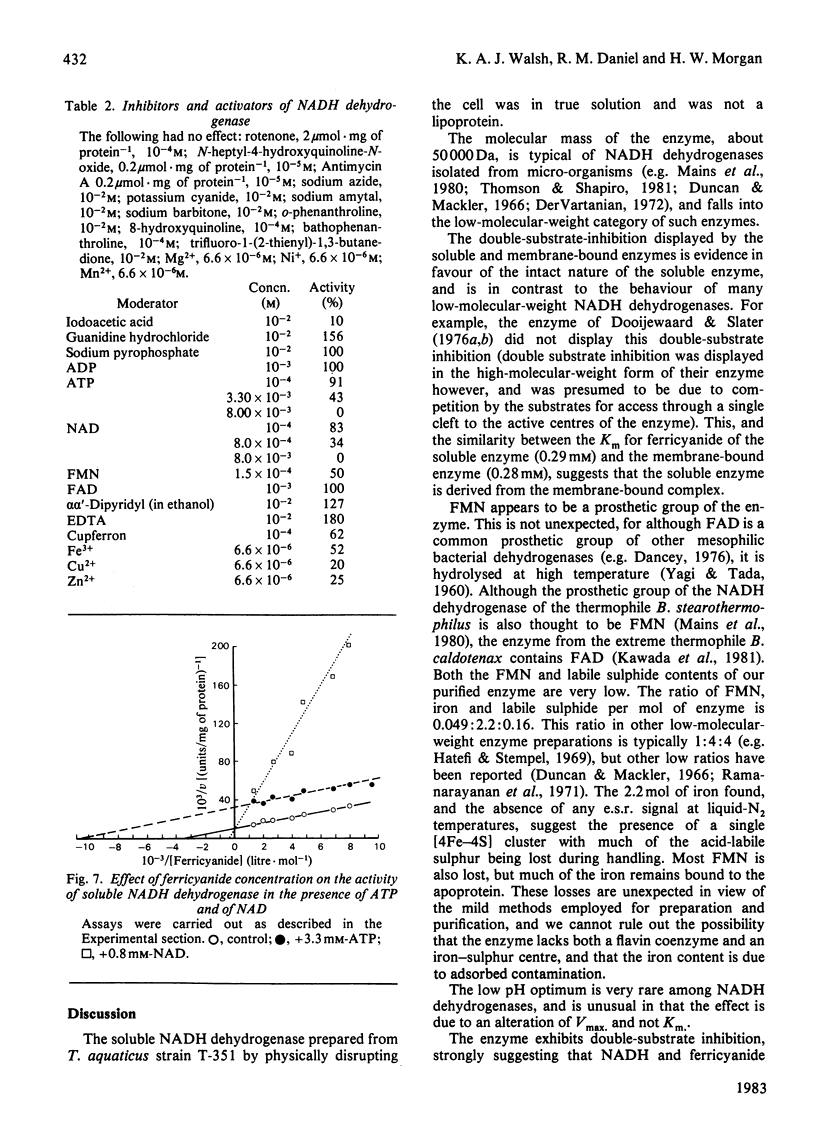
A soluble NADH dehydrogenase (NADH:ferricyanide oxidoreductase) has been obtained by simple disruption of cells of Thermus aquaticus strain T351, and purified. The enzyme is of low molecular mass, 50 000 Da, and displays many of the properties of the membrane-bound enzyme, including inhibition by both NADH and ferricyanide, and the same Km for ferricyanide. The enzyme contains 0.05 mol of FMN, 0.16 mol of labile sulphur and 2.2 mol of iron per mol of protein. The enzyme is inhibited by NAD and cupferron competitively with ferricyanide, and by ATP (but not ADP) competitively with NADH. The enzyme is particularly thermostable, having a half-life at 95 degrees C of 35 min. The effect of temperature on the molar absorption coefficient and the stability of NADH was determined.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amelunxen R., Lins M. Comparative thermostability of enzymes from Bacillus stearothermophilus and Bacillus cereus. Arch Biochem Biophys. 1968 Jun;125(3):765–769. doi: 10.1016/0003-9861(68)90512-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Dooijewaard G., Slater E. C. Steady-state kinetics of high molecular weight (type-I) NADH dehydrogenase. Biochim Biophys Acta. 1976 Jul 9;440(1):1–15. doi: 10.1016/0005-2728(76)90109-2. [DOI] [PubMed] [Google Scholar]
- Dooijewaard G., Slater E. C. Steady-state kinetics of low molecular weight (type-II) NADH dehydrogenase. Biochim Biophys Acta. 1976 Jul 9;440(1):16–35. doi: 10.1016/0005-2728(76)90110-9. [DOI] [PubMed] [Google Scholar]
- Duncan H. M., Mackler B. Electron-transport systems of yeast. II. Purification and properties of a soluble reduced diphosphopyridine nucleotide dehydrogenase. Biochemistry. 1966 Jan;5(1):45–50. doi: 10.1021/bi00865a007. [DOI] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Hatefi Y., Stempel K. E. Isolation and enzymatic properties of the mitochondrial reduced diphosphopyridine nucleotide dehydrogenase. J Biol Chem. 1969 May 10;244(9):2350–2357. [PubMed] [Google Scholar]
- Hirata H., Sone N., Yoshida M., Kagawa Y. Isolation of the alanine carrier from the membranes of a thermophilic bacterium and its reconstitution into vesicles capable of transport. J Supramol Struct. 1977;6(1):77–84. doi: 10.1002/jss.400060106. [DOI] [PubMed] [Google Scholar]
- Hirata H., Sone N., Yoshida M., Kagawa Y. Solubilization and partial purification of alanine carrier from membranes of a thermophilic bacterium and its reconstitution into functional vesicles. Biochem Biophys Res Commun. 1976 Apr 5;69(3):665–671. doi: 10.1016/0006-291x(76)90927-x. [DOI] [PubMed] [Google Scholar]
- KOFFLER H., GALE G. O. The relative thermostability of cytoplasmic proteins from thermophilic bacteria. Arch Biochem Biophys. 1957 Mar;67(1):249–251. doi: 10.1016/0003-9861(57)90267-9. [DOI] [PubMed] [Google Scholar]
- Kawada N., Nosoh Y. Relation of Arrhenius discontinuities of NADH dehydrogenase to change in membrane lipid fluidity of Bacillus caldotenax. FEBS Lett. 1981 Feb 9;124(1):15–18. doi: 10.1016/0014-5793(81)80043-9. [DOI] [PubMed] [Google Scholar]
- Kawada N., Takeda K., Nosoh Y. Effect of lipids on a membrane-bound NADH dehydrogenase from Bacillus caldotenax. J Biochem. 1981 Apr;89(4):1017–1027. [PubMed] [Google Scholar]
- Mains I., Power D. M., Thomas E. W., Buswell J. A. Purification of an NADH-(dichlorophenol-indophenol) oxidoreductase from Bacillus stearothermophilus. Biochem J. 1980 Nov 1;191(2):457–465. doi: 10.1042/bj1910457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McComb R. B., Bond L. W., Burnett R. W., Keech R. C., Bowers G. N., Jr Determination of the molar absorptivity of NADH. Clin Chem. 1976 Feb;22(2):141–150. [PubMed] [Google Scholar]
- Ramanarayanan M., Rao N. A., Vaidyanathan C. S. Purification & properties of a soluble reduced nicotinamide adenine dinucleotide (NADH) dehydrogenase from Agrobacterium tumefaciens. Indian J Biochem. 1971 Dec;8(4):214–218. [PubMed] [Google Scholar]
- Thomson J. W., Shapiro B. M. The respiratory chain NADH dehydrogenase of Escherichia coli. Isolation of an NADH:quinone oxidoreductase from membranes and comparison with the membrane-bound NADH:dichlorophenolindophenol oxidoreductase. J Biol Chem. 1981 Mar 25;256(6):3077–3084. [PubMed] [Google Scholar]
- Tipton K. F., Dixon H. B. Effects of pH on enzymes. Methods Enzymol. 1979;63:183–234. doi: 10.1016/0076-6879(79)63011-2. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wisdom C., Welker N. E. Membranes of Bacillus stearothermophilus: factors affecting protoplast stability and thermostability of alkaline phosphatase and reduced nicotinamide adenine dinucleotide oxidase. J Bacteriol. 1973 Jun;114(3):1336–1345. doi: 10.1128/jb.114.3.1336-1345.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


