Abstract
Background
Data on the impact of valve position on clinical outcomes in patients with atrial fibrillation (AF) and bioprosthetic valves (BPVs) are limited.
Methods and Results
The BPV-AF Registry was a multicenter, prospective, observational study involving 894 patients with BPVs and AF. In this post-hoc substudy, patients were classified according to BPV position: aortic (n=588; 65.8%), mitral (n=195; 21.8%), or both (n=111; 12.4%). The primary outcome was a composite of stroke/systemic embolism, major bleeding, heart failure requiring hospitalization, all-cause death, or BPV reoperation. During a mean follow up of 15.3±4.0 months, the primary outcome occurred in 90 (15.3%) patients (12.7/100 patient-years) in the aortic group, 25 (12.8%; 10.2/100 patient-years) in the mitral group, and 16 (14.4%; 11.8/100 patient-years) in the both-valves group (log-rank P=0.621). The unadjusted and adjusted risks were not significant for the mitral and both-valves groups relative to the aortic group (unadjusted hazard ratio [95% confidence interval] 0.80 [0.52–1.25] and 0.92 [0.54–1.57]; adjusted hazard ratio 0.89 [0.51–1.54] and 1.10 [0.58–2.09], respectively). There was no significant difference in the incidence of stroke/systemic embolism or major bleeding among the 3 groups (log-rank P=0.651 and 0.156, respectively).
Conclusions
In patients with BPVs and AF, the risk for the composite outcome was comparable regardless of the BPV position.
Key Words: Atrial fibrillation, Bioprosthetic valve, Bleeding, Thromboembolism
With the rapid progression of an aging society, the number of patients with atrial fibrillation (AF) is increasing.1,2 Additionally, there has been a rise in the use of bioprosthetic valves (BPVs) for valve replacement because of significant valvular heart disease, driven by the improved durability of BPVs and the expansion of transcatheter interventions.3,4 Therefore, the coexistence of AF and BPV implantation is a growing concern, and risk stratification for patients with these overlapping conditions is crucial for the appropriate treatment, including antithrombotic therapy.5–7
The position and number of implanted valves significantly influence patient characteristics and outcomes, even after treating the underlying valvular heart disease with valve replacements. In patients with or without AF who have undergone BPV implantation, those with a BPV in the mitral position have a higher thromboembolic risk than those with a BPV in the aortic position.8 Additionally, patients undergoing double valve replacement have a higher perioperative risk than those undergoing isolated valve replacement.9 However, data on outcomes after the perioperative period in patients with both aortic and mitral BPVs and AF remain sparse.
We previously reported that the bleeding risk in patients with AF and BPVs in the mitral position was higher than that in the aortic position, while the thromboembolic risk was comparable between the two groups.10 However, because of the limited number of patients, we could not include patients with AF who had BPVs in both the aortic and mitral positions. Therefore, this study was performed to evaluate the influence of valve position in patients with AF and BPVs, including the mitral, aortic, and both valves, using a large prospective registry in Japan.
Methods
Study Population
The BPV-AF Registry was a multicenter, prospective, and observational study designed to clarify antithrombotic therapy and outcomes in patients with BPV replacement and AF in real-world clinical practice in Japan. Briefly, 894 patients who had undergone BPV replacement at least 3 months before enrollment and had been diagnosed with AF were included from 16 hospitals in Japan between September 2018 and October 2019. The enrolled patients were followed up for a minimum of 1 year (until October 2020). The study design and main results of the BPV-AF Registry have been published elsewhere.11,12 The study was conducted in compliance with the Declaration of Helsinki, the Ethical Guidelines for Medical and Health Research Involving Human Subjects, and all other applicable regulatory and legal requirements. The protocol and informed consent document were reviewed and approved by the Ethics Committee of the National Cerebral and Cardiovascular Center (M30-068; September 26, 2018) and each participating hospital (UMIN000034485).
In this post-hoc substudy, all enrolled patients were divided into 3 groups according to the position of the BPVs: aortic valve, mitral valve, or both valves. The aortic valve group included patients who underwent both surgical aortic valve replacement and transcatheter aortic valve implantation (TAVI). Baseline characteristics and clinical outcomes were compared across the 3 groups.
Clinical Outcomes
The primary outcome of the present study was defined as a composite of stroke/systemic embolism, major bleeding (based on the International Society on Thrombosis and Haemostasis criteria13), heart failure requiring hospitalization, all-cause death, or BPV reoperation, consistent with our previous substudy of patients with a BPV in the aortic position.14 The secondary outcomes were stroke/systemic embolism and major bleeding. Other outcomes for each component of the primary outcome were also presented. Detailed definitions of each event have been published previously.11,12
Statistical Analysis
Continuous variables are presented as mean±standard deviation or median and interquartile range, and were compared using analysis of variance or the Kruskal-Wallis test depending on the distribution. Categorical variables are presented as number and percentage and were compared using the chi-squared test. The incidence rates for all outcomes were calculated per 100 person-years (PY). The cumulative incidences of the primary and secondary outcomes were calculated using the Kaplan-Meier method, and differences were assessed with the log-rank test. Cox proportional hazard regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the outcomes, comparing the mitral valve and both-valves groups with the aortic valve group as the reference. In the multivariable Cox proportional hazards regression models, the following risk-adjusting variables were included to estimate HRs and 95% CIs: each component of the CHA2DS2-VASc score (heart failure, hypertension, age, diabetes, ischemic stroke, vascular disease, and sex) was incorporated as a separate risk-adjusting variable. Additionally, body mass index, estimated glomerular filtration rate, type of AF, direct oral anticoagulants (DOACs), antiplatelet therapy, and left ventricular ejection fraction were also included, considering clinical relevance in accordance with our previous report.14 Multivariable models were not constructed for outcomes with fewer than 20 events because they were insufficient for reliable models. All statistical analyses were performed using SAS (version 9.4; SAS Institute Inc., Cary, NC, USA). All reported P values are 2-tailed, and P<0.05 was considered statistically significant.
Results
Baseline and Operative Characteristics
Among the 894 patients, 588 (65.8%) had a BPV in the aortic valve position, 195 (21.8%) in the mitral valve position, and 111 (12.4%) in both valve positions. The baseline characteristics of each group based on the valve position are shown in Table 1. Patients in the aortic valve group were more often male, older, and had a higher body weight. They also had higher CHADS2, CHA2DS2-VASc, and HAS-BLED scores. The prevalence of paroxysmal AF was higher in the aortic valve group than in the other groups. Regarding medical histories and comorbidities, patients in the mitral valve group had ischemic stroke less often, while patients in the aortic valve group had hypertension, dyslipidemia, and peripheral arterial disease more often. Patients in the aortic valve group had a higher left ventricular ejection fraction but smaller left atrial diameter, left atrial volume, and left atrial volume index.
Table 1.
Baseline Characteristics
| Aortic valve (n=588) |
Mitral valve (n=195) |
Both valves (n=111) |
P value | |
|---|---|---|---|---|
| Male | 287 (48.8) | 77 (39.5) | 46 (41.4) | 0.047 |
| Age (years) | 81.5±6.7 | 77.8±7.3 | 78.6±6.2 | <0.001 |
| Weight (kg) | 54.7±11.9 | 52.1±10.5 | 52.1±9.3 | 0.012 |
| BMI (kg/m2) | 22.6±3.9 | 21.3±3.1 | 21.6±2.8 | <0.001 |
| CHADS2 score | 2.7±1.2 | 2.2±1.2 | 2.3±1.1 | <0.001 |
| ≥2.0 | 456 (85.2) | 133 (71.1) | 84 (76.4) | <0.001 |
| CHA2DS2-VASc score | 4.3±1.5 | 3.8±1.3 | 3.9±1.3 | <0.001 |
| ≥3.0 | 484 (89.8) | 158 (84.5) | 91 (82.7) | 0.039 |
| HAS-BLED score | 2.6±1.1 | 2.2±1 | 2.3±1 | <0.001 |
| ≥3.0 | 265 (49.6) | 65 (35.1) | 44 (40.4) | 0.002 |
| eGFR (mL/min/1.73 m2) | 45.1±17.5 | 48.8±17.6 | 51.4±17.3 | <0.001 |
| Type of AF | ||||
| Paroxysmal | 261 (44.4) | 52 (26.7) | 19 (17.1) | <0.001 |
| Persistent | 205 (34.9) | 67 (34.4) | 38 (34.2) | |
| Permanent | 122 (20.8) | 76 (39.0) | 54 (48.7) | |
| Previous history of CVD | ||||
| Ischemic stroke | 88 (15.0) | 16 (8.2) | 19 (17.1) | 0.033 |
| Hemorrhagic stroke | 14 (2.4) | 4 (2.1) | 3 (2.7) | 0.933 |
| Intracranial hemorrhage | 17 (2.9) | 11 (5.6) | 2 (1.8) | 0.113 |
| Systemic embolism | 7 (1.2) | 2 (1.0) | 2 (1.8) | 0.830 |
| Major bleeding | 24 (4.1) | 16 (8.2) | 7 (6.3) | 0.071 |
| Comorbidities | ||||
| Hypertension | 495 (84.2) | 111 (56.9) | 76 (68.5) | <0.001 |
| Heart failure | 328 (55.8) | 105 (53.9) | 59 (53.2) | 0.818 |
| Dyslipidemia | 319 (54.3) | 79 (40.5) | 45 (40.5) | 0.001 |
| Diabetes | 121 (20.6) | 42 (21.5) | 26 (23.4) | 0.788 |
| Renal dysfunction | 60 (10.2) | 17 (8.7) | 7 (6.3) | 0.406 |
| Chronic respiratory disease | 58 (9.9) | 19 (9.7) | 9 (8.1) | 0.846 |
| Malignant tumor | 52 (8.8) | 9 (4.6) | 7 (6.3) | 0.133 |
| Vascular disease | 70 (11.9) | 14 (7.2) | 4 (3.6) | 0.010 |
| Myocardial infarction | 31 (5.3) | 12 (6.2) | 2 (1.8) | 0.222 |
| Peripheral arterial disease | 29 (4.9) | 2 (1.0) | 2 (1.8) | 0.023 |
| Aortic plaque | 16 (2.7) | 3 (1.5) | 0 (0.0) | 0.154 |
| Thrombosis and embolism | 17 (2.9) | 5 (2.6) | 6 (5.4) | 0.331 |
| Echocardiography parameters | ||||
| LVEF (%) | 61.9±10.6 | 56.3±11.7 | 56.6±12.8 | <0.001 |
| <40 | 25 (4.6) | 18 (10.0) | 13 (12.4) | <0.001 |
| 40–49 | 39 (7.1) | 24 (13.3) | 10 (9.5) | |
| ≥50 | 484 (88.3) | 138 (76.7) | 82 (78.1) | |
| LVEDD | 44.7±7.1 | 47.5±7.9 | 45.8±6.7 | <0.001 |
| LVESD | 29.5±7.5 | 33.0±8.7 | 31.7±6.8 | <0.001 |
| LAD | 46.8±8.1 | 53.2±11.4 | 53.2±11.9 | <0.001 |
| LA volume | 103.7±43.5 | 137.6±90.9 | 145.8±83.9 | <0.001 |
| LA volume index (LA volume/BSA) | 69.7±28.3 | 96.9±65.4 | 102.8±59.1 | <0.001 |
Categorical variables are presented as n (%). Continuous variables are presented as mean±SD. AF, atrial fibrillation; BMI, body mass index; BSA, body surface area; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; LA, left atrium; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter.
Regarding operative characteristics, patients in the aortic group underwent BPV replacement mainly for treatment of aortic stenosis, with 40.1% of patients undergoing TAVI. Patients in the aortic group underwent surgery with concurrent left atrial plication or left atrial appendage occlusion/excision less often (Table 2).
Table 2.
Operative Characteristics
| Aortic valve (n=588) |
Mitral valve (n=195) |
Both valves (n=111) | ||
|---|---|---|---|---|
| Aortic valve | Mitral valve | |||
| VHD subtype | ||||
| Stenosis | 445 (75.7) | 81 (41.5) | 69 (62.2) | 56 (50.5) |
| Regurgitation | 114 (19.4) | 95 (48.7) | 33 (29.7) | 43 (38.7) |
| Other | 29 (4.9) | 19 (9.7) | 9 (8.1) | 12 (10.8) |
| Operation type | ||||
| Surgery | 352 (59.9) | 195 (100.0) | 111 (100.0) | 111 (100.0) |
| TAVI | 236 (40.1) | – | 0 (0.0) | – |
| History of replacement | ||||
| First replacement | 561 (95.4) | 171 (87.7) | 100 (90.1) | |
| Re-replacement | 25 (4.3) | 24 (12.3) | 11 (9.9) | |
| Left atrial plication, LAA occlusion/excision | 49 (8.4) | 34 (17.5) | 20 (18) | |
Categorical variables are presented as n (%). LAA, left atrial appendage; TAVI, transcatheter aortic valve implantation; VHD, valvular heart disease.
The administration status of antithrombotic agents is presented in Table 3. DOAC-based therapy was received more frequently in the aortic valve group (221 [37.6%] patients) than in the mitral valve and both-valves groups (31 [15.9%] and 11 [9.9%], respectively).
Table 3.
Administration Status of Antithrombotic Agents (Anticoagulant and Antiplatelet Drugs)
| Aortic valve (n=588) |
Mitral valve (n=195) |
Both valves (n=111) |
P value | |
|---|---|---|---|---|
| No antithrombotic drug | 40 (6.8) | 11 (5.6) | 5 (4.5) | 0.605 |
| Warfarin-based therapy | n=258 | n=144 | n=87 | |
| No antiplatelet drug | 175 (67.8) | 114 (79.2) | 63 (72.4) | 0.052 |
| With antiplatelet drug | 83 (32.2) | 30 (20.8) | 24 (27.6) | |
| With aspirin (monotherapy) | 70 (27.1) | 27 (18.8) | 23 (26.4) | 0.156 |
| With P2Y12 (monotherapy) | 11 (4.3) | 1 (0.7) | 0 (0.0) | 0.023 |
| Prasgrel | 2 | 0 | 0 | |
| Clopidogrel | 8 | 1 | 0 | |
| Ticlopidine | 1 | 0 | 0 | |
| With DAPT | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| With others | 2 (0.8) | 2 (1.4) | 1 (1.2) | 0.835 |
| DOAC-based therapy | n=221 | n=31 | n=11 | |
| No antiplatelet drug | 157 (71.0) | 25 (80.7) | 7 (63.6) | 0.444 |
| With antiplatelet drug | 64 (29.0) | 6 (19.4) | 4 (36.4) | |
| With aspirin (monotherapy) | 46 (20.8) | 5 (16.1) | 4 (36.4) | 0.365 |
| With P2Y12 (monotherapy) | 16 (7.2) | 0 (0.0) | 0 (0.0) | 0.198 |
| Prasgrel | 2 | 0 | 0 | |
| Clopidogrel | 14 | 0 | 0 | |
| Ticlopidine | 0 | 0 | 0 | |
| With DAPT | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| With others | 2 (0.9) | 1 (3.2) | 0 (0.0) | 0.489 |
| Antiplatelet therapy (without warfarin/DOAC) | n=69 | n=9 | n=8 | |
| Aspirin (monotherapy) | 53 (76.8) | 8 (88.9) | 6 (75.0) | 0.698 |
| P2Y12 (monotherapy) | 10 (14.5) | 1 (11.1) | 0 (0.0) | 0.503 |
| Prasgrel | 0 | 0 | 0 | |
| Clopidogrel | 10 | 1 | 0 | |
| Ticlopidine | 0 | 0 | 0 | |
| DAPT | 4 (5.8) | 0 (0.0) | 0 (0.0) | 0.596 |
| Prasgrel | 0 | 0 | 0 | |
| Clopidogrel | 4 | 0 | 0 | |
| Ticlopidine | 0 | 0 | 0 | |
| With others | 2 (2.9) | 0 (0.0) | 2 (25.0) | 0.015 |
Categorical variables are presented as n (%). DAPT, dual-antiplatelet therapy; DOAC, direct oral anticoagulants.
Clinical Outcomes
During the mean follow-up period of 15.3±4.0 months, the primary outcome was observed in 90 (15.3%) patients (12.7/100 PY) in the aortic valve group, 25 (12.8%) patients (10.2/100 PY) in the mitral valve group, and 16 (14.4%) patients (11.8/100 PY) in the both-valves group (Table 4). The cumulative incidence of the primary outcome was not significantly different among the 3 groups (log-rank P=0.621; Figure A). The Cox proportional hazards regression models for the primary outcome showed no significant difference in the mitral valve and both-valves groups relative to the aortic valve group (unadjusted HR 0.80, 95% CI 0.52–1.25, P=0.333, and adjusted HR 0.89, 95% CI 0.51–1.54, P=0.669 in the mitral valve group; unadjusted HR 0.92, 95% CI 0.54–1.57, P=0.768, and adjusted HR 1.10, 95% CI 0.58–2.09, P=0.773 in the both-valves group; Table 4).
Table 4.
Clinical Outcomes
| Outcome / Valve position | No. patients with event (%) |
Per 100 PY |
Unadjusted HR (95% CI) |
P value | Adjusted HR (95% CI) |
P value |
|---|---|---|---|---|---|---|
| Composite outcome | ||||||
| Aortic | 90 (15.3) | 12.7 | Ref. | – | Ref. | – |
| Mitral | 25 (12.8) | 10.2 | 0.80 (0.52–1.25) | 0.333 | 0.89 (0.51–1.54) | 0.669 |
| Both | 16 (14.4) | 11.8 | 0.92 (0.54–1.57) | 0.768 | 1.10 (0.58–2.09) | 0.773 |
| Stroke/systemic embolism | ||||||
| Aortic | 14 (2.4) | 1.90 | Ref. | – | Ref. | – |
| Mitral | 4 (2.1) | 1.59 | 0.84 (0.28–2.56) | 0.761 | 0.54 (0.13–2.13) | 0.375 |
| Both | 4 (3.6) | 2.90 | 1.55 (0.51–4.72) | 0.437 | 0.50 (0.09–2.72) | 0.424 |
| Stroke | ||||||
| Aortic | 12 (2.0) | 1.62 | Ref. | – | – | – |
| Mitral | 3 (1.5) | 1.19 | 0.74 (0.21–2.61) | 0.637 | – | – |
| Both | 3 (2.7) | 2.16 | 1.36 (0.38–4.80) | 0.638 | – | – |
| Systemic embolism | ||||||
| Aortic | 2 (0.3) | 0.27 | Ref. | – | – | – |
| Mitral | 1 (0.5) | 0.40 | 1.49 (0.14–16.38) | 0.747 | – | – |
| Both | 1 (0.9) | 0.72 | 2.73 (0.25–30.06) | 0.413 | – | – |
| Major bleeding | ||||||
| Aortic | 17 (2.9) | 2.31 | Ref. | – | Ref. | – |
| Mitral | 1 (0.5) | 0.40 | 0.17 (0.02–1.30) | 0.088 | 0.14 (0.02–1.22) | 0.075 |
| Both | 3 (2.7) | 2.15 | 0.94 (0.28–3.21) | 0.922 | 0.44 (0.09–2.32) | 0.335 |
| HF requiring hospitalization | ||||||
| Aortic | 42 (7.1) | 5.79 | Ref. | – | Ref. | – |
| Mitral | 18 (9.2) | 7.32 | 1.26 (0.72–2.19) | 0.415 | 1.73 (0.85–3.53) | 0.131 |
| Both | 4 (3.6) | 2.90 | 0.49 (0.18–1.37) | 0.176 | 1.00 (0.34–2.96) | 1.000 |
| All-cause death | ||||||
| Aortic | 33 (5.6) | 4.42 | Ref. | – | Ref. | – |
| Mitral | 8 (4.1) | 3.17 | 0.71 (0.33–1.54) | 0.387 | 0.73 (0.29–1.83) | 0.504 |
| Both | 6 (5.4) | 4.30 | 0.97 (0.41–2.31) | 0.938 | 1.19 (0.42–3.35) | 0.749 |
| BPV reoperation | ||||||
| Aortic | 5 (0.9) | 0.67 | Ref. | – | – | – |
| Mitral | 1 (0.5) | 0.40 | 0.60 (0.07–5.13) | 0.640 | – | – |
| Both | 1 (0.9) | 0.72 | 1.08 (0.13–9.24) | 0.945 | – | – |
BPV, bioprosthetic valve; CI, confidence interval; HF, heart failure; HR, hazard ratio; PY, patient-years.
Figure.
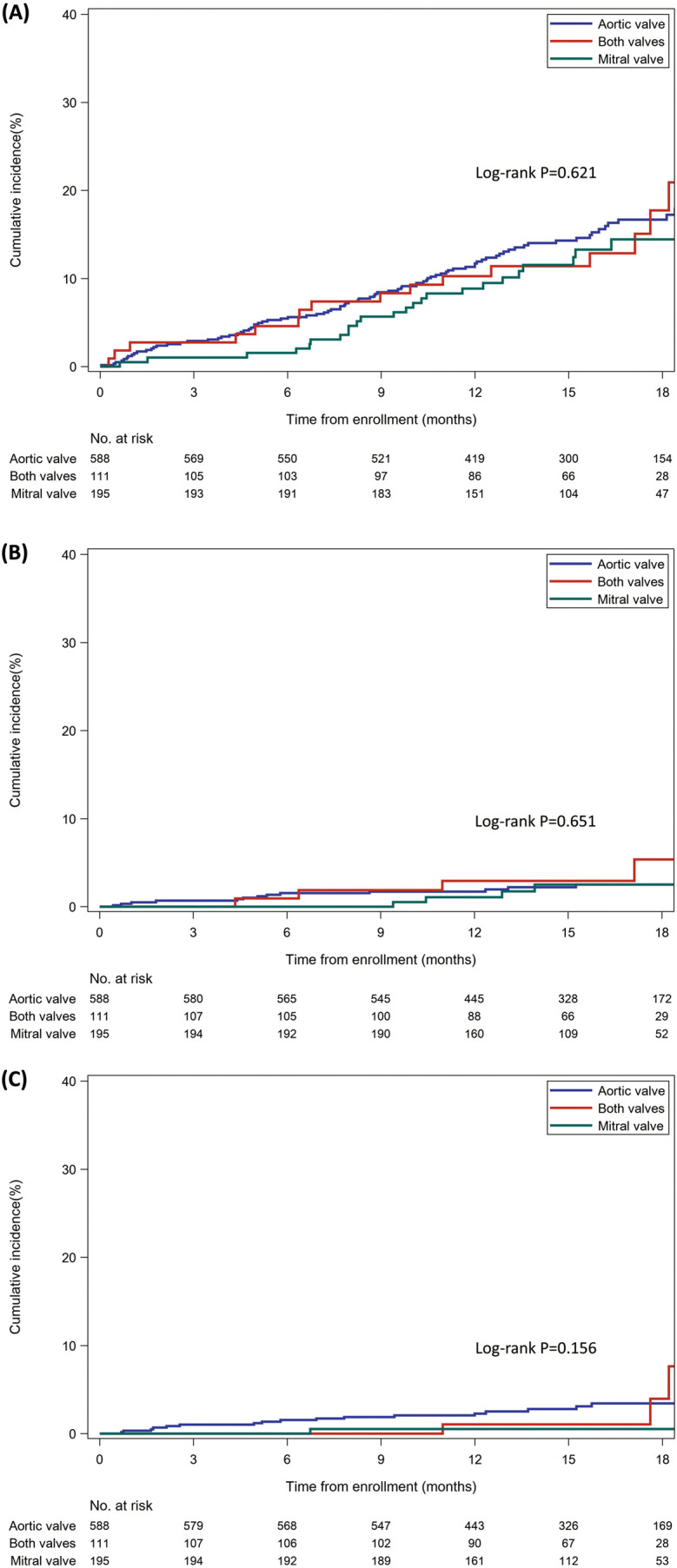
Kaplan-Meier curves for clinical outcomes according to bioprosthetic valve (BPV) positions. (A) Composite outcome (stroke, systemic embolism, major bleeding, heart failure requiring hospitalization, all-cause death, or BPV reoperation). (B) Stroke or systemic embolism. (C) Major bleeding.
The incidence of stroke/systemic embolism was numerically higher in the both-valves group (3.6%; 2.90/100 PY) than in the aortic valve group (2.4%; 1.90/100 PY) and the mitral valve group (2.1%; 1.59/100 PY; Table 4). However, there was no significant difference in the cumulative incidence of stroke/systemic embolism among the 3 groups (log-rank P=0.651; Figure B). The unadjusted and adjusted HRs in the mitral valve group and the both-valves group relative to the aortic valve group were also not significantly different (unadjusted HR 0.84, 95% CI 0.28–2.56, P=0.761, and adjusted HR 0.54, 95% CI 0.13–2.13, P=0.375 in the mitral valve group; unadjusted HR 1.55, 95% CI 0.51–4.72, P=0.437, and adjusted HR 0.50, 95% CI 0.09–2.72, P=0.424 in the both-valves group; Table 4).
Major bleeding was observed in 17 (2.9%) patients (2.31/100 PY) in the aortic valve group, 1 (0.5%) patient (0.40/100 PY) in the mitral valve group, and 3 (2.7%) patients (2.15/100 PY) in the both-valves group (Table 4). Although the incidence of major bleeding was numerically lower in the mitral valve group, the cumulative incidence of major bleeding was not significantly different among the 3 groups with the limited number of events (log-rank P=0.156; Figure C). Both the unadjusted and adjusted HRs in the mitral valve group were not significantly lower than those in the aortic valve group (unadjusted HR 0.17, 95% CI 0.02–1.30, P=0.088, and adjusted HR 0.14, 95% CI 0.02–1.22, P=0.075; Table 4).
Discussion
The 2 main findings of the present study involving patients with BPVs and AF are as follows. First, the risk for the composite endpoint was comparable regardless of whether patients had BPVs in the aortic valve, mitral valve, or both valve positions. Second, the thromboembolic and bleeding risks did not significantly differ based on the valve position.
Generally, patients who undergo surgical valve replacement in the mitral position are considered to have a higher risk of thromboembolic events than those who undergo surgical valve replacement in the aortic position. For example, the recommended prothrombin time-international normalized ratio for patients with a mechanical valve is higher in the mitral position than in the aortic position.5–7 Additionally, a previous study suggested that the thromboembolic risk of patients with a BPV in the mitral position was higher than in the aortic position, partly because of the higher prevalence of AF.8 Furthermore, patients who underwent double valve replacement have an increased thromboembolic risk, especially in the perioperative period.9 However, in the present study, no significant difference in the cumulative incidence of stroke/systemic embolism was observed based on valve position, and the valve position itself was not independently associated with the thromboembolic risk (although the incidence of stroke/systemic embolism was numerically higher in the both-valves group). In patients with BPVs and AF, AF may have a greater influence on thromboembolic events than BPVs. In fact, in the Fushimi AF Registry, a community-based AF population, the 1-year incidence of stroke or systemic embolism was 2.7%;15 this was even higher than in our registry. This may suggest that regardless of the BPV position, we can utilize risk stratification parameters similar to those used in the general AF population, such as the CHA2DS2-VASc score, for patients with BPVs and AF.6
In terms of bleeding risk, patients with a BPV in the aortic position reportedly have a higher cumulative incidence of bleeding events than those in the mitral position, reflecting the older population of patients undergoing surgical aortic valve implantation.8 In addition, the mean age of patients undergoing aortic valve replacement has been increasing with the widespread use of TAVI.16 In this study, patients with a BPV in the aortic valve position were older and had a higher HAS-BLED score than those with a BPV in the mitral and both valve positions, partly because patients who underwent TAVI comprised approximately 40% of the aortic valve group. Nevertheless, there was no significant difference in the cumulative incidence of major bleeding among the 3 groups. This may be attributed to comparable baseline risk factors for bleeding among the 3 groups, such as body weight and a history of major bleeding, although our previous report indicated that patients with a BPV and AF in the mitral position had a higher rate of major bleeding due to differences in these factors.10
The lack of a significant difference in the composite outcome as well as each component among the 3 groups suggests that the impact of valve position was mitigated by the presence of AF. The exclusion of patients who underwent BPV replacement within 3 months may have also contributed to the similar cumulative incidences in the both-valves group compared with those in the aortic and mitral valve groups. Therefore, valve position alone may be insufficient for risk stratification in patients with BPVs and AF in the chronic phase, and a similar anticoagulation strategy may be considered regardless of valve position. Several randomized trials involving patients with AF and BPVs stratified by each valve position have shown that DOACs were non-inferior to warfarin. In the RIVER trial, which included patients with AF and a BPV in the mitral position, rivaroxaban was found to be non-inferior to warfarin for the composite outcome.17 In patients with AF who underwent TAVI, edoxaban was also non-inferior to warfarin for a composite of adverse events.18 Additionally, our previous study, which included patients with AF and a BPV in the aortic position, suggested that the effect of DOACs vs. warfarin did not significantly differ between surgical aortic valve replacement and TAVI.14 However, no definitive evidence is currently available for patients with AF and BPVs in both valve positions. To establish an appropriate antithrombotic strategy in patients with AF and BPVs in both valve positions, further research is warranted to identify the risk factors for these patients.
Study Limitations
This study has several limitations. First, despite the increased number of enrolled patients compared with our previous study, the follow-up period was relatively short (median of 15.3 months). Additionally, the low event rate for each endpoint resulted in a limited number of events overall. Thus, this study might be too underpowered to precisely evaluate the impact of valve position in this population. Second, we excluded patients who had undergone BPV replacement within 3 months to evaluate antithrombotic therapy in a stable phase. Therefore, the high-risk features in patients with double valve replacement, especially in the early phase, were not reflected. Furthermore, unlike in the main study, we were unable to compare DOAC-based and warfarin-based antithrombotic therapy for each valve position.12 Third, both the presence of BPVs and the presence of AF were influential factors for thromboembolic events. Although the mechanisms and etiology of thromboembolism may differ, we cannot clearly differentiate whether stroke/systemic embolism originated from BPVs or AF. Fourth, considering the observational nature of this study and the substantial differences in the baseline characteristics among the 3 groups, the possibility of unmeasured confounders in estimating the risk of clinical events cannot be ruled out, despite the intensive multivariable adjustments.
Conclusions
In patients with AF who had undergone BPV replacement at least 3 months prior, the risk for the composite outcome was similar regardless of whether the BPV was positioned in the aortic valve, mitral valve, or both valves. Appropriate risk stratification and management would be necessary for patients with AF and BPVs, irrespective of the valve position.
Sources of Funding
This study was supported by Daiichi Sankyo Co., Ltd (Tokyo, Japan) in collaboration with the National Cerebral and Cardiovascular Center.
Disclosures
H.T. has received remuneration from AstraZeneca plc, Ono Pharmaceutical Company, Limited, Pfizer Inc, Otsuka Pharmaceutical Co., Ltd., Daiichi Sankyo Company, Limited, Eli Lilly and Company, Boehringer Ingelheim GmbH, Abbott Japan LLC, and Novartis International AG. K.A. has received remuneration from Japan Lifeline, Abbott Medical Japan, Medtronic Japan, BIOTRONIK Japan, TERUMO, and Novartis Pharma. M.I. has received consultancy fees from Abbott Medical Japan LLC, and remuneration from Edwards Lifesciences Corporation. T. Kimura has received remuneration from Abbott Medical Japan LLC, research funding from Research Institute for Production Development, EP-CRSU Co., Ltd, Edwards Lifesciences Corporation, and Kowa Pharmaceutical Co., Ltd, and scholarship funds or donations from Nippon Boehringer Ingelheim Co., Ltd, Otsuka Pharmaceutical Co., Ltd, Daiichi Sankyo Co., Ltd, Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Company Limited, Bayer Yakuhin Ltd, and Research Institute for Production Development. T.S. has received remuneration from Medtronic Japan Co., Ltd. K.M. has received scholarship funds or donations from Edwards Lifesciences Corporation, Terumo Co., Ltd, and Japan Lifeline Co., Ltd. K.T. has received remuneration from Amgen K.K., Bayer Yakuhin Ltd, Daiichi Sankyo Co., Ltd, Kowa Pharmaceutical Co. Ltd, Novartis Pharma K.K., Otsuka Pharmaceutical Co., Ltd, and Pfizer Japan Inc., research funding from AMI Co., Ltd, Bayer Yakuhin Ltd, Bristol-Myers Squibb K.K., EA Pharma Co., Ltd, and Mochida Pharmaceutical Co., Ltd, scholarship funding from AMI Co., Ltd, Bayer Yakuhin Ltd, Nippon Boehringer Ingelheim Co., Ltd, Chugai Pharmaceutical Co., Ltd, Daiichi Sankyo Co., Ltd, Edwards Lifesciences Corporation, Johnson & Johnson K.K., Ono Pharmaceutical Co., Ltd, Otsuka Pharmaceutical Co., Ltd, and Takeda Pharmaceutical Co., Ltd, and is affiliated with the endowed department sponsored by Abbott Japan Co., Ltd, Boston Scientific Japan K.K., Fides-one Inc., GM Medical Co., Ltd, ITI Co., Ltd, Kaneka Medix Co., Ltd, Nipro Corporation, Terumo Co., Ltd, Abbott Medical Co., Ltd, Cardinal Health Japan LLC, Fukuda Denshi Co., Ltd, Japan Lifeline Co., Ltd, Medical Appliance Co., Ltd, and Medtronic Japan Co., Ltd. Y. Sakata has received remuneration from Daiichi Sankyo Co., Ltd, and Nippon Boehringer Ingelheim Co., Ltd, and scholarship funding from Nippon Boehringer Ingelheim Co., Ltd, Bayer Yakuhin Ltd, and Daiichi Sankyo Co., Ltd. M.F. is an employee of Daiichi Sankyo Co., Ltd. T.T. has received remuneration from Daiichi Sankyo Co., Ltd, and Bayer Yakuhin Ltd. K.N. has received research funding from Philips Japan Ltd, Terumo Co., Ltd, TEPCO Power Grid Inc., and Asahi Kasei Pharma Co. Y.F. has received remuneration from Daiichi Sankyo Co., Ltd, Bayer Yakuhin Ltd, and Novartis Pharma K.K. C.I. has received remuneration and research funding from Daiichi Sankyo Co., Ltd. K.T. is a member of Circulation Reports’ Editorial Team. The other authors have no conflicts of interest to disclose.
IRB Information
The protocol and the informed consent document were reviewed and approved by the Ethics Committee of the National Cerebral and Cardiovascular Center (M30-068; September 26, 2018). The main study was registered in the UMIN Clinical Trials Registry under Identifier No. UMIN000034485.
Acknowledgments
The authors thank the staff and participants of the BPV-AF Registry for their significant contributions to this work. During the preparation of this manuscript, the authors used ChatGPT-4o for English language proofreading only.
Data Availability
The deidentified participant data that underlie the results reported in the present study will be shared on request immediately after publication until 36 months post-publication. The study protocol will also be available. Researchers requesting data should provide a methodologically sound proposal detailing how the data will be used; this proposal may be reviewed by responsible personnel at Daiichi Sankyo Co., Ltd. Data will be provided to achieve the aims of the approved proposal. Please contact the corresponding author directly to request data sharing. Data requestors will need to sign a data access agreement.
References
- 1. Inoue H, Fujiki A, Origasa H, Ogawa S, Okumura K, Kubota I, et al.. Prevalence of atrial fibrillation in the general population of Japan: An analysis based on periodic health examination. Int J Cardiol 2009; 137: 102–107. [DOI] [PubMed] [Google Scholar]
- 2. Lane DA, Skjøth F, Lip GYH, Larsen TB, Kotecha D.. Temporal trends in incidence, prevalence, and mortality of atrial fibrillation in primary care. J Am Heart Assoc 2017; 6: e005155, doi:10.1161/JAHA.116.005155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Culler SD, Cohen DJ, Brown PP, Kugelmass AD, Reynolds MR, Ambrose K, et al.. Trends in aortic valve replacement procedures between 2009 and 2015: Has transcatheter aortic valve replacement made a difference? Ann Thorac Surg 2018; 105: 1137–1143. [DOI] [PubMed] [Google Scholar]
- 4. Chan J, Narayan P, Fudulu DP, Dong T, Angelini GD.. Trend in mitral valve prostheses of choice and early outcomes in the United Kingdom. Int J Cardiol 2024; 397: 131607, doi:10.1016/j.ijcard.2023.131607. [DOI] [PubMed] [Google Scholar]
- 5. Izumi C, Eishi K, Ashihara K, Arita T, Otsuji Y, Kunihara T, et al.. JCS/JSCS/JATS/JSVS 2020 guidelines on the management of valvular heart disease. Circ J 2020; 84: 2037–2119. [DOI] [PubMed] [Google Scholar]
- 6. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, et al.. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021; 143: e72–e227, doi:10.1161/CIR.0000000000000923. [DOI] [PubMed] [Google Scholar]
- 7. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al.. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2022; 43: 561–632. [DOI] [PubMed] [Google Scholar]
- 8. Heras M, Chesebro JH, Fuster V, Penny WJ, Grill DE, Bailey KR, et al.. High risk of thromboemboli early after bioprosthetic cardiac valve replacement. J Am Coll Cardiol 1995; 25: 1111–1119. [DOI] [PubMed] [Google Scholar]
- 9. Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Bärwolf C, Levang OW, et al.. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on valvular heart disease. Eur Heart J 2003; 24: 1231–1243. [DOI] [PubMed] [Google Scholar]
- 10. Obayashi Y, Miyake M, Amano M, Kitai T, Takegami M, Nishimura K, et al.. Impact of mitral versus aortic bioprosthetic valve position on thromboembolism and bleeding risk in patients with atrial fibrillation. J Cardiol 2022; 79: 226–232. [DOI] [PubMed] [Google Scholar]
- 11. Furukawa Y, Miyake M, Fujita T, Koyama T, Takegami M, Kimura T, et al.. Rationale, design, and baseline characteristics of the BioProsthetic Valves with Atrial Fibrillation (BPV-AF) Study. Cardiovasc Drugs Ther 2020; 34: 689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Izumi C, Miyake M, Fujita T, Koyama T, Tanaka H, Ando K, et al.. Antithrombotic therapy for patients with atrial fibrillation and bioprosthetic valves: Real-world data from the multicenter, prospective, observational BPV-AF Registry. Circ J 2022; 86: 440–448. [DOI] [PubMed] [Google Scholar]
- 13. Schulman S, Kearon C.. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3: 692–694. [DOI] [PubMed] [Google Scholar]
- 14. Miyake M, Takegami M, Obayashi Y, Amano M, Kitai T, Fujita T, et al.. Comparison of direct oral anticoagulants and warfarin in patients with atrial fibrillation and an aortic bioprosthetic valve. Circ J 2022; 86: 1699–1707. [DOI] [PubMed] [Google Scholar]
- 15. Akao M, Abe M, Chun YH, Esato M, Tsuji H, Wada H, et al.. Inappropriate use of oral anticoagulants for patients with atrial fibrillation: 1-year outcomes of the Fushimi AF Registry. Circ J 2014; 78: 2166–2172. [DOI] [PubMed] [Google Scholar]
- 16. Nguyen V, Willner N, Eltchaninoff H, Burwash IG, Michel M, Durand E, et al.. Trends in aortic valve replacement for aortic stenosis: A French nationwide study. Eur Heart J 2022; 43: 666–679. [DOI] [PubMed] [Google Scholar]
- 17. Guimarães HP, Lopes RD, de Barros E Silva PGM, Liporace IL, Sampaio RO, Tarasoutchi F, et al.. Rivaroxaban in patients with atrial fibrillation and a bioprosthetic mitral valve. N Engl J Med 2020; 383: 2117–2126. [DOI] [PubMed] [Google Scholar]
- 18. Van Mieghem NM, Unverdorben M, Hengstenberg C, Möllmann H, Mehran R, López-Otero D, et al.. Edoxaban versus vitamin K antagonist for atrial fibrillation after TAVR. N Engl J Med 2021; 385: 2150–2160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The deidentified participant data that underlie the results reported in the present study will be shared on request immediately after publication until 36 months post-publication. The study protocol will also be available. Researchers requesting data should provide a methodologically sound proposal detailing how the data will be used; this proposal may be reviewed by responsible personnel at Daiichi Sankyo Co., Ltd. Data will be provided to achieve the aims of the approved proposal. Please contact the corresponding author directly to request data sharing. Data requestors will need to sign a data access agreement.


