Abstract
To address the current use of high-concentration (70–75%) alcohol solutions as disinfectants, which are known for their drawbacks such as flammability and strong odor, a new approach based on nanosecond pulse-driven bubble discharge in low-concentration ethanol solutions is proposed. Research findings indicate that O2 bubble plasma activated ethanol solution (PAES) exhibits superior sterilization efficacy. A 3 min treatment using 10% alcohol eliminated all bacteria (reducing the bacterial count by 7 orders of magnitude) with an energy requirement of only 10.9 J/ml, whereas the same treatment with air PAES achieved less than a one-order reduction and O2/N2/air plasma activated water (PAW) achieved even less. Furthermore, to delve deeper into the key factors of PAES sterilization, concentrations of inorganic reactive species (H2O2, NO2–, NO3–, ONOO–, pH) and organic components resulting from alcohol decomposition (CH3CHO, CH3CH2OH, CH3COOH, and CH3COOOH) were analyzed using assay kits and GC-MS. Their variations at different storage temperatures (4 °C and −20 °C) were compared with the corresponding bactericidal effects. The results identified peroxyacetic acid (CH3COOOH) as a key bactericidal factor in PAES, showing that CH3COOOH over time at different storage temperatures correlated with their bactericidal effects. The study also revealed that O2 PAES stored at −20 °C maintained complete bacterial elimination even after 4 days. Therefore, PAES has the potential to replace high-concentration alcohol solutions for sterilization.
1. Introduction
Since the end of 2019, COVID-19 has swept across the world, marking the worst global infectious pandemic in a century. Thanks to the efforts of people worldwide, the World Health Organization declared on May 5, 2023, that COVID-19 no longer constituted a “Public Health Emergency of International Concern.″ However, the threat of infectious diseases persists, with ailments such as COVID-19, influenza, and norovirus remaining prevalent in winter and spring.
One of the primary means of transmission of pathogenic bacteria is contact transmission, a process through which pathogens come into direct or indirect contact via a medium. Ethanol with a 75% volume fraction is the most common contact transmission blocker on the market. Despite its prevalence, it has several disadvantages, including:
(i) An irritating odor that may cause allergies.
(ii) A strong stinging sensation when in contact with open wounds.
(iii) Flammability, necessitating safe storage.
Furthermore, ethanol only exhibits bactericidal effects within the 70% to 75% volume fraction range, due to its similar osmotic pressure to that of bacteria in this concentration.1 Consequently, the need for new, environmentally friendly, efficient, and safe disinfectants is imminent.
Plasma activated water (PAW) has gained increasing attention as a disinfectant in recent years due to its safe and gentle characteristics.2−4 However, some problems exist in the PAW field. The solution system formed after plasma treatment consists of species with a lifespan ranging from microseconds to seconds.5 These short-lived species are converted into more stable and long-lived compounds, such as H2O2, NO2–, NO3–, after the plasma is off. These species will continue to react to form NO3– until one of the precursors is exhausted. This results in only few stable reactive species left in the PAW.2
To address this issue, a disinfectant based on a plasma activated ethanol solution (PAES) was proposed in our previous work.6 By introducing ethanol into the plasma-activated liquid phase system, air plasma can react not only with water to produce reactive oxygen and nitrogen species (RONS: OH, NO2–, NO3–, H+, H2O2, O3, O2–, ONOO–) but also with ethanol to produce more complex reactive components. These reactive components can further react with the RONS produced by the plasma-water reaction, potentially resulting in more reactive ingredients with higher bactericidal efficiency. As shown in our previous work, air plasma activation of a 10% ethanol solution for 2 min can completely kill bacteria (a 7-log reduction), whereas the same plasma treatment of water can only achieve a 1-log reduction.
On the other hand, although our previous work demonstrated that PAES has significant advantages over PAW, achieving efficient sterilization, two key issues still need to be addressed. First, the key reactive components responsible for the bactericidal effect in PAES remain unclear. Second, the efficiency of PAES production was not very high in previous studies. Therefore, this study aims to address these two issues, as follows:
(i) For the key issue of the key reactive species responsible for the bactericidal effect in PAES are still not clear from previous work, this study addresses this critical scientific question by utilizing different working gases and quantitatively measuring various reactive species in the corresponding PAES using various assay kits and Gas Chromatography–Mass Spectrometry (GC-MS), further comparing them with the bactericidal efficiency, and ultimately understanding the key factors for bactericidal action in PAES.
(ii) Based on the research results of the first part, to improve the efficiency of PAES production, this study approaches the problem from two aspects. First, from the perspective of plasma generation. Previously, when plasma was generated above an ethanol solution, a large amount of short-lived species dissipated in the air before they could react with the ethanol solution. In this study, plasma is generated within the ethanol solution, enabling the active species to immediately react with the ethanol after being generated.
Thus, to enhance plasma activity, a nanosecond-pulsed power supply was chosen to drive the bubble discharge in the ethanol solution. Compared with AC or DC discharges, nanosecond pulse-driven discharge has high breakdown voltage and high electron energy. Ultrafast rising edge on the nanosecond scale are capable of generating fast ionization waves and high reduced electric field.7 The electron energy is significantly increased to achieve efficient ionization of the gas which results in a highly reactive plasma and a better performance in bactericidal, wastewater treatment and so on. The needle-mesh electrode discharge system was chosen because the needle electrode can create the strongest electric field with the same applied voltage.8
Moreover, this study found that using oxygen instead of air for discharge in the ethanol solution significantly improved bactericidal effects by several orders of magnitude, thus oxygen was used as the working gas. Ultimately, it was found that both the plasma treatment volume and energy efficiency increased by more than an order of magnitude.
2. Materials and Methods
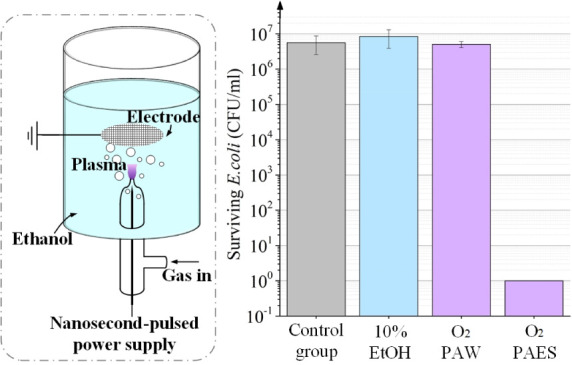
2.1. Nanosecond-Pulsed Plasma Activated Ethanol Solution System
The nanosecond-pulsed plasma-activated ethanol solution (PAES) system used in this study is shown in Figure 1. A high-voltage tungsten needle electrode was placed in a quartz tube (OD 12 mm, ID 10 mm). The nozzle of the quartz tube had an outer diameter of 3 mm and an inner diameter of 1 mm, with the tip of the tungsten needle positioned 2 mm inside the nozzle. A 40-holes-per-inch multihollow titanium mesh electrode was used as the ground electrode, positioned 2 cm from the nozzle. This device processed 35 mL of solution at a time and was powered by a nanosecond-pulsed power supply. Air, oxygen, or nitrogen was introduced into the quartz tube at a flow rate of 0.5 standard liters per minute (slm).
Figure 1.
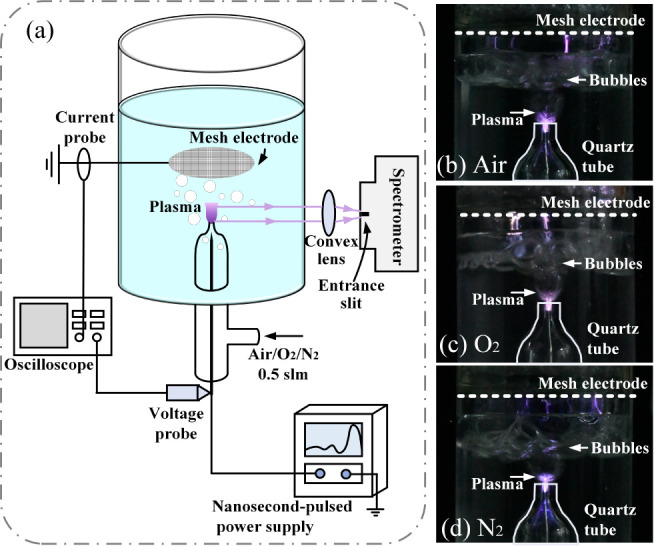
Experiment setup and the photos of the plasma. (a) Schematic of experiment setup, and photos of (b) air plasma, (c) O2 plasma, and (d) N2 plasma.
A voltage probe (Tektronix P6015A) was used to measure the voltage applied to the HV electrode, while a current probe (Tektronix TCP312A) measured the current flowing through the plasma. The waveforms of voltage and current under the three different gases are shown in Figure 2.
Figure 2.
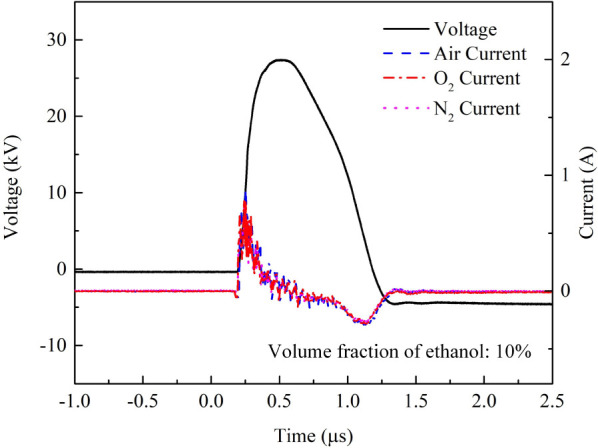
Typical currents and voltage waveforms of the plasma for different working gases.
The voltage applied in this research is 27 kV, with a pulse width of 300 ns and a frequency of 1 kHz. The waveforms and peak currents are essentially the same for all three gases (air, O2, and N2), with the peak current around 800 mA. The electric power deposited into the plasma remains nearly constant regardless of the type of gas used. It can be calculated using the formula below:
| 1 |
Here, Pdis is the power consumed by the plasma, f is the discharge frequency, V(t) is the applied voltage, I(t) is the discharge current, τ is the pulse-width.
2.2. Optical Emission Spectrum of the Plasma
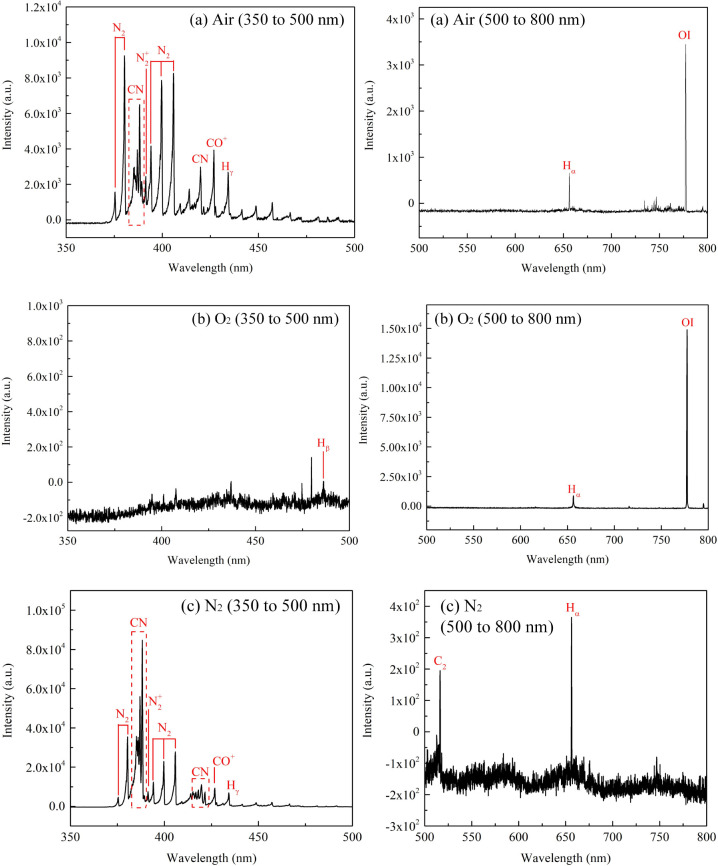
A spectrometer (Princeton Instruments Acton SpectraHub 2500i) was used to measure the emission spectrum of air, O2, and N2 plasma in an ethanol solution. The results are shown in Figure 3. The optical emission spectrum acquisition system is shown in Figure 1a. A convex lens is placed between the plasma and the spectrometer at an appropriate position to focus the plasma radiation into the entrance slit of the spectrometer. Both the entrance and exit slits were set at 200 μm wide. The grating was set to 1200 grooves per millimeter. The step size was set at 0.1 nm. Moreover, a filter is placed in front of the entrance slit of the spectrometer to eliminate the interference of spectral lines below 500 nm when collecting spectral lines in the wavelength range of 500 nm–800 nm.
Figure 3.
Optical emission spectra of the plasma for different working gases (volume fraction of ethanol: 10%). (a) air, (b) O2, (c) N2.
Since the vessel used to hold the PAES is made of acrylic material, it can only transmit light above approximately 350 nm. Therefore, the measured spectra range from 350 to 800 nm.
When ethanol was treated with air plasma or N2 plasma, as shown in Figure 3a,c, the spectra of CN and CO appeared. The spectrum of C2 appeared when N2 was used as the working gas.
When O2 was used as the working gas, as shown in Figure 3b, only the spectral lines of Hα, Hβ, and O atoms were detected.
2.3. Reactive Species Measurement
The volume of the treated solution is 35 mL. The ethanol (Sinopharm Chemical Reagent, China) was diluted to different volume fractions with distilled water for further study. PAES was quickly shaken well after preparation. And PAES or the control solution was immediately sampled for active inorganic and organic reactive species detection. The concentrations of the NO2–, NO3– were measured using a nitrite assay kit. A total nitric oxide assay kit, based on the Griess method, was also used. An ultraviolet spectrophotometer was used to read the absorbance at a wavelength of 550 nm. The concentration of H2O2 was measured using a H2O2 assay kit, with the results detected by a microplate reader at a wavelength of 560 nm. The pH value was measured with a pH probe, and the conductivity was measured with a conductivity meter. Details about the detection methods can be found in our previous study.6
The analysis of organic active species in PAES was performed using GC-MS (Shimadzu, Japan). The GC-MS parameters were set as follows: Chromatography Column: Rtx-5MS, 30 m × 0.25 mm × 0.25 μm; interface temperature: 250 °C; column temperature: 50 °C; ion source temperature: 200 °C; split ratio: 50; column flow rate: 1 mL/min; carrier gas: He, 36 cm/s, constant velocity.
2.4. Bacterial Analysis
E. coli was selected to evaluate the bactericidal effects of PAES. E. coli (ATCC25922) was provided by the College of Life Science and Technology, HUST. The initial concentration of the E. coli solution was 107 CFU/ml.
One ml of the E. coli solution was centrifuged at 10,000 r/min for 10 min, and the supernatant was carefully removed, leaving only the sediment. PAES was quickly shaken well after preparation. And 1 mL of PAES or the control solution was immediately taken and mixed thoroughly with the sediment. The mixture was left to stand for 15 min. The bacterial solution was then smeared on Tryptose Soya Agar (TSA) media, which was placed in a bacterial incubator for approximately 24 h for further analysis.
3. Results and Discussion
3.1. Bactericidal Effects of PAES
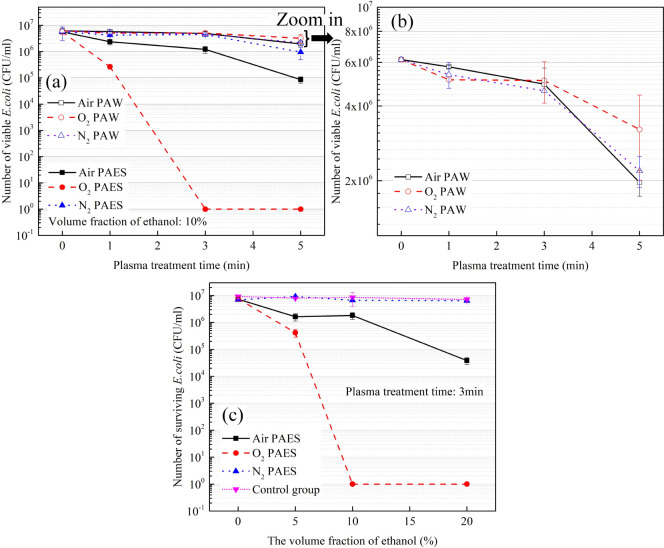
The bactericidal effect of PAES was investigated by varying the plasma working gas, treatment time, and the volume fraction of ethanol. For comparison, the experimental results of plasma-activated water (PAW) are also included. The results are shown in Figure 4.
Figure 4.
(a) The bactericidal effect of PAES and PAW using air, O2, and N2 as working gases with different plasma treatment times. (b) The zoomed-in view of bactericidal effect of PAW using air, O2, and N2 as working gases with different plasma treatment times. (c)The bactericidal effect of PAES using different working gases (air, O2, N2) and various ethanol volume fractions.
As shown in Figure 4a, the bactericidal effect of O2 PAES is stronger than that of air PAES and N2 PAES. N2 PAES can only kill 1 log of bacteria, and air PAES can kill 2 logs with 5 min of plasma treatment. In contrast, O2 PAES can achieve a 7-log reduction with just 3 min of plasma treatment.
The bactericidal effects of the air, O2, and N2 PAW are shown in the Figure 4b. All three of them has a poor bactericidal effect, failing to kill even 1 log of bacteria. And the bactericidal effect of the PAW prepared under the three working gases differed very little. This experiment strongly demonstrates the higher activity of PAES compared to PAW.
Next, the plasma treatment time was set to 3 min to explore the effect of the volume fraction of ethanol on the bactericidal effect of PAES. The results are shown in Figure 4c. The control group consisted of ethanol at corresponding volume fractions without plasma treatment. The results proved that the control group and N2 PAES did not have bactericidal ability. In contrast, O2 plasma-activated 10% ethanol solution completely killed the bacteria, while air plasma-activated 10% ethanol could only kill less than 1 log of bacteria. Even with 20% ethanol, air PAES could only kill less than 3 logs of bacteria.
In terms of energy efficiency, this study demonstrates that using an O2 plasma for 3 min can activate 10% alcohol to achieve complete bacterial elimination. Our plasma power was about 2.1 W, and the treated alcohol volume was 35 mL, resulting in an energy requirement of only 10.9J/ml to achieve sterilization. In contrast, previous reports indicated that a plasma power of 2.0 W was needed to treat 2 mL of alcohol, requiring 120 J/ml to achieve the same effect.6
3.2. Long-Lived Species in PAES
The concentrations of long-lived RONS (NO2–, NO3–, H2O2) in PAES treated with different gas plasmas were measured.
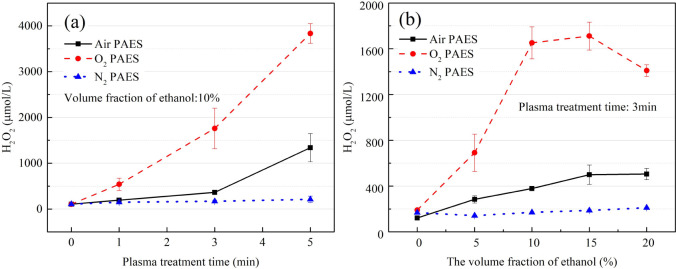
The concentration of H2O2 varying with plasma treatment time is shown in Figure 5a. The H2O2 concentration in N2 PAES remained extremely low regardless of the plasma treatment time. In contrast, the H2O2 concentrations in O2 PAES and air PAES increased with plasma treatment time, with O2 PAES reaching up to 3800 μmol/L within 5 min of treatment.
Figure 5.
(a) The concentration of H2O2 in PAES with air/O2/N2 as working gas, varying with plasma-treatment time; (b) the concentration of H2O2 in PAES with air/O2/N2 as working gas, varying with the volume fraction of ethanol.
The concentration of H2O2 varying with the volume fraction of ethanol is shown in Figure 5b. H2O2 in O2 PAES showed a rising trend with increasing ethanol volume fraction, peaking at 1711 μmol/L at 15% ethanol before decreasing. A similar, albeit less pronounced, trend was observed in air PAES. Adding appropriate amount of ethanol facilitates the production of H2O2. However, ethanol-excessive environment can cause the rapid hydrogen abstractions of ethanol in reaction (11)11 to scavenge OH radicals.9
There appears to be a correlation between the concentration of H2O2 and the bactericidal effect of PAES. O2 PAES, which had the best bactericidal effect, also contained the highest concentration of H2O2. This outcome suggests that may contribute to the high activity of PAES.
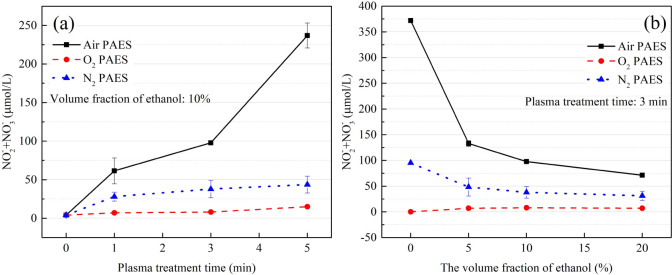
The concentration of NOx for different working gases, varying with plasma treatment time, is shown in Figure 6a. NOx levels are much higher in air PAES than in O2 PAES and N2 PAES. In O2 PAES, NOx levels are almost negligible regardless of the treatment time. However, NOx levels increase in air PAES and N2 PAES with treatment time.
Figure 6.
(a) The concentration of NOx in PAES with air, O2, and N2 as working gases, varying with plasma treatment time. (b) The concentration of NOx in PAES with air, O2, and N2 as working gases, varying with the volume fraction of ethanol.
The concentration of NOx for different working gases, varying with the volume fraction of ethanol, is shown in Figure 6b. NOx levels in air PAES and N2 PAES continued to decrease as the volume fraction of ethanol increased, finally leveling off when the volume fraction reached 10%. Almost no NOx was detected in O2 PAES.
These results indicate that NOx is not the key species responsible for the bactericidal effect of PAES.
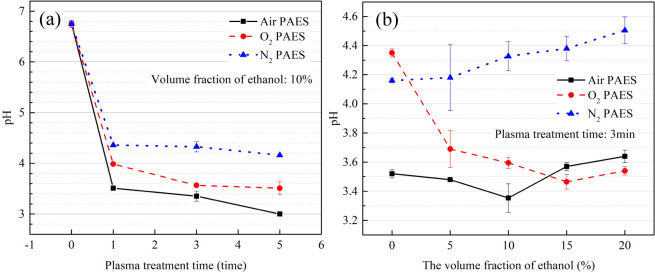
The pH of PAES decreases with increasing treatment time, as shown in Figure 7a. Air PAES has the lowest pH, while N2 PAES has the highest pH.
Figure 7.
(a) The pH in PAES with air, O2, and N2 as working gases, varying with plasma-treatment time. (b) The pH in PAES with air, O2, and N2 as working gases, varying with the volume fraction of ethanol.
There is a slight upward trend in pH for air PAES and N2 PAES with increasing ethanol volume fraction, as shown in Figure 7b. In contrast, there is a significant decrease in pH for O2 PAES, which levels off when the ethanol volume fraction reaches 10%.
3.3. Bactericidal Effects and Chemical Properties of PAES During Storage
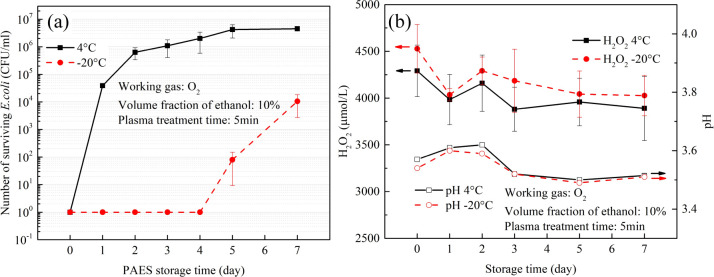
O2 PAES (with 10% ethanol) treated for 5 min was chosen to study the storage characteristics of PAES due to its good bactericidal properties and high H2O2 concentration.
Two storage temperatures were set: 4 °C and −20 °C. The stored PAES was then used to treat bacteria, and the survival numbers are shown in Figure 8a. After 1 day of storage at 4 °C, the bactericidal property of PAES was greatly reduced, killing only 2 orders of magnitude of bacteria. In contrast, PAES stored at −20 °C could kill 7 orders of magnitude of bacteria even after 4 days. It is evident that the activity of PAES is well preserved when stored at −20 °C.
Figure 8.
Effects of storage time and temperature on PAES: (a) bactericidal efficacy, and (b) H2O2 concentration and pH value.
The changes in H2O2 concentration and pH value of PAES are shown in Figure 8b. As seen in Figure 8b, the pH remained basically unchanged whether stored at 4 °C or −20 °C. After 7 days of storage, the concentration of H2O2 decreased from 4500 to 4000 μmol/L at 4 °C and from 4300 to 3900 μmol/L at −20 °C. There was not much discrepancy in the concentration of H2O2 between the two storage conditions. It is evident that the change in H2O2 concentration is not the main factor causing the difference in the bactericidal effect of PAES at different storage temperatures.
3.4. The Analysis of Organic Reactive Species
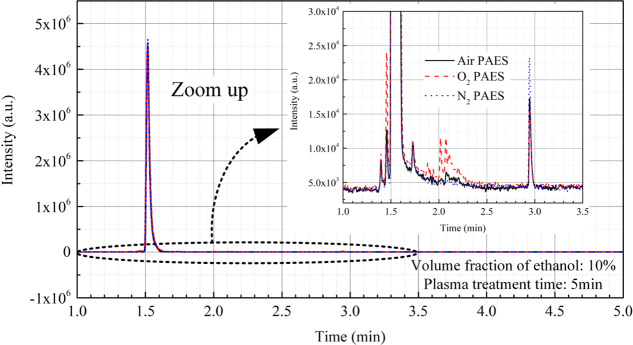
The PAES were analyzed by GC-MS, and the resulting gas chromatograms are shown in Figure 9. The O2 PAES exhibited a series of new peaks in the retention time range of 1.8 to 2.5 min compared to N2 PAES and air PAES. These new peaks might be related to the key reactive species to the excellent bactericidal effect of O2 PAES.
Figure 9.
GC-MS measurement results of the PAES for air/O2/N2 plasma.
The mass spectrum of each peak in the chromatogram was analyzed, and the composition corresponding to each peak was identified. The results are shown in Table 1. By analyzing these components, it was found that only ethanol, hydrogen peroxide, acetic acid, and peroxyacetic acid have bactericidal properties and are used for sterilization. Therefore, one of these compounds or their synergistic effect is responsible for the bactericidal properties of PAES. The mass spectra of the important peaks in O2 PAES are shown in Figure 10.
Table 1. Component of the PAES Detected by GC-MS.
| Retention time (min) | Species | Formula |
|---|---|---|
| 1.4 | Oxygen | O2 |
| 1.453 | Acetaldehyde | CH3CHO |
| 1.517 | Ethanol | CH3CH2OH |
| 1.730 | 1-Propanol | CH3CH2CH2OH |
| 1.887, 1.940 | Butane-1,4-diol | C4H10O2 |
| 2.017 | Hydrogen peroxide | H2O2 |
| 2.073 | Acetic acid | CH3COOH |
| 2.1–2.2 | Peroxyacetic acid | CH3COOOH |
| 2.947 | Ethane, 1,1-diethoxy- | C6H14O2 |
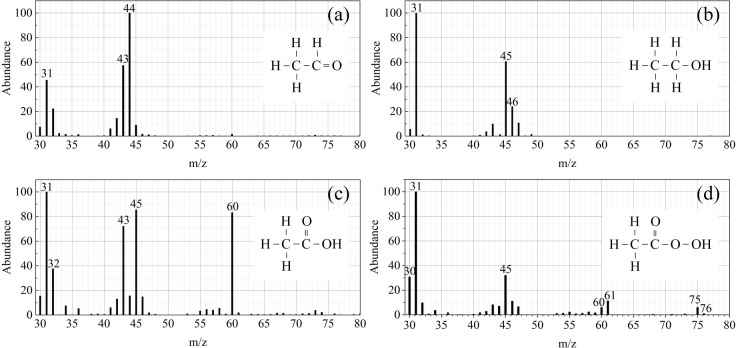
Figure 10.
Mass spectra of peaks which are important in O2 PAES, (a) acetaldehyde(CH3CHO), (b) ethanol(CH3CH2OH), (c) acetic acid(CH3COOH), and (d) peroxyacetic acid(CH3COOOH).
The external standard method was used to calibrate the concentrations of CH3CH2OH, CH3COOH and CH3COOOH in the air/O2/N2 PAES (5 min, 10%) as shown in Table 2. The concentrations of H2O2 were derived from the data in Figure 5a and the unit has been transformed into ppm.
Table 2. Concentrations of the Key Components of Air/O2/N2 PAES.
| Concentration |
||||
|---|---|---|---|---|
| Species | Ethanol (10%volume fraction) | Air PAES | O2 PAES | N2 PAES |
| CH3CH2OH | 78 927 ppm | 66 775 ppm | 74 254 ppm | 68 478 ppm |
| H2O2 | 3.6 ppm | 45.56 ppm | 130 ppm | 7.2 ppm |
| CH3COOH | 0 ppm | 8.63 ppm | 41 ppm | 2.16 ppm |
| CH3COOOH | 0 ppm | 27.94 ppm | 166.16 ppm | 0 ppm |
Peroxyacetic acid (CH3COOOH) might be the most critical component determining the high activity of PAES. The reasons for this are as follows: CH3COOOH is a strong oxidant with a high oxidation potential and a relatively high reactive oxygen content (21.1%). It can disrupt sulfhydryl (−SH) and disulfide (S–S) bonds in proteins and enzymes, leading to DNA double-strand unraveling and breakage. CH3COOOH is widely used for disinfection and bleaching.
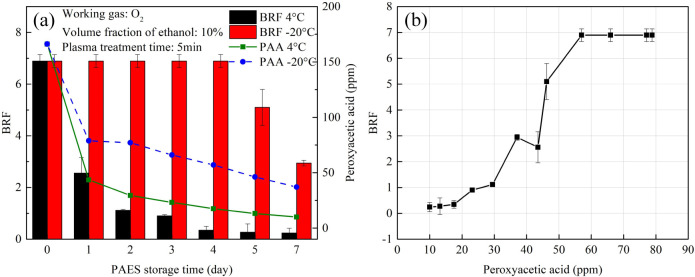
The bactericidal results in Figure 8a were converted to the Bacterial Reduction Factor (BRF) to better understand the correlation between the bactericidal capacity of O2 PAES and the concentration of peroxyacetic acid. The BRF was calculated as follows:
| 2 |
It can be observed that the change in the bactericidal capacity of O2 PAES closely follows the trend of peroxyacetic acid (CH3COOOH) concentration over different storage times (Figure 11a). The BRF and CH3COOOH concentration data from Figure 11a were plotted, with BRF as the vertical axis and CH3COOOH concentration as the horizontal axis, resulting in Figure 11b. It is clear that BRF and CH3COOOH are correlated until CH3COOOH reaches approximately 60 ppm, at which point it is able to kill all the bacteria. This outcome indicates that CH3COOOH plays a significant role in the bactericidal properties of PAES.
Figure 11.
(a)The correlation between the bactericidal capacity of O2 PAES under different temperature with the change of the PAES storage time (peracetic acid is abbreviated as PAA). (b)The correlation between the bactericidal capacity of O2 PAES and the concentration of the peracetic acid.
In our previous work,6 it was speculated that the combined effects of pH, CH3COOOH, H2O2, CH3COOH, and even ONOO– contributed to the high bactericidal activity of PAES. The production rate of ONOO– is determined by the concentrations of H2O2, NO2–, and H+.10 However, NO2– in O2 PAES is due to the extremely small amount of air dissolved in the water and is barely detectable. As a result, the high activity of PAES can exclude the effects of ONOO–.
To further understand the mechanism of PAES, a series of chemical solutions containing one or more of H+, H2O2, CH3COOH, and CH3COOOH were prepared to test their bactericidal effects, as shown in Table 3. The concentrations of these components were consistent with their corresponding concentrations in O2 PAES. The pH was set to 3, matching that of O2 PAES, which corresponds to an H+ concentration of 0.001%.
Table 3. Groups of the Manually Configured Solutions and PAES.
| Group | Content |
|---|---|
| A | H2O2 |
| B | CH3COOH |
| C | CH3COOH+ H2O2 |
| D | CH3COOH+ H2O2+H+ |
| E | Peroxyacetic acid disinfectant |
| F | O2 PAES |
In group E, a commercially available peroxyacetic acid(CH3COOOH) disinfectant was diluted until the concentration of CH3COOOH was consistent with that in O2 PAES (10% ethanol, 5 min plasma treatment). It should be emphasized that the peroxyacetic acid disinfectant contains acetic acid and hydrogen peroxide in addition to peroxyacetic acid. Their concentrations were also measured, resulting in group E containing peroxyacetic acid: 166 ppm, acetic acid: 445 ppm, hydrogen peroxide: 118 ppm, and a pH of 3.
The bactericidal results are shown in Figure 12. It is evident that the concentrations of H2O2 and CH3COOH in groups A and B are insufficient to effectively kill bacteria. Group D, which was manually configured to include the potential key ingredients (H+, H2O2, and CH3COOH), was only able to reduce bacterial count by 1 log. Therefore, none of these components alone are critical for bactericidal activity.
Figure 12.
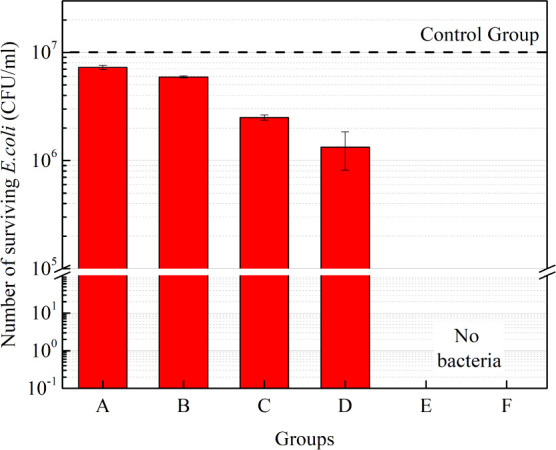
Bactericidal effects of different chemical mixtures and O2 PAES.
Comparing group E to group F, the only significant difference is the concentration of acetic acid. Results from group B indicate that such a low concentration of acetic acid has negligible bactericidal effect. Thus, by comparing groups D, E, and F, it can be concluded that peroxyacetic acid(CH3COOOH) is indeed the key component in O2 PAES.
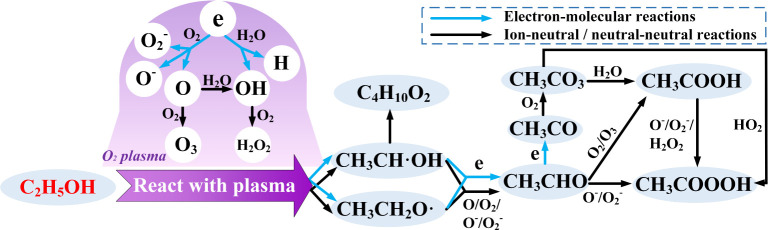
3.5. The Reactions in O2 PAES System
The complex interaction between O2 plasma and ethanol solution has been studied to elucidate the mechanisms in the O2 PAES system. The electrons, ions, and radicals generated by plasma act similarly to catalysts, participating in reactions and facilitating or accelerating various reaction pathways.11
Electron impact dissociation of O2 can produce a significant amount of ROS in the gas-phase of plasma region, as demonstrated in reactions 33 and 4.12 Additionally, electrons can be readily absorbed by O2 to form O2– due to the high electron affinity of O2, as shown in reaction 55.13
| 3 |
| 4 |
| 5 |
O atoms can react with water to form OH in the gas-phase of plasma region and the gas–liquid interface, as shown in reaction 66. In an O2-dominated plasma system, the production rate of highly reactive OH is very high. And these OH can diffuse into the liquid and quenched quickly within a very thin layer of the liquid surface.9,14
| 6 |
H2O and C2H5OH cannot dissociate to form gaseous H or OH through thermal decomposition at room temperature. However, they can interact with electrons generated by oxygen plasma to form reactive species in the gas-phase of plasma region, as shown in reactions 77–10. C2H5O radicals are crucial for the production of CH3COOOH, a key component in the PAES system. C2H5O radicals exist in three main isomers: (CH3-·CH–OH(84.3%), ·CH2–CH2–OH(13.2%), CH3–CH2–O·(2.5%)).15
| 7 |
| 8 |
| 9 |
| 10 |
OH can abstract hydrogen from ethanol to form C2H5O radicals in the gas-phase and interface, as shown in reaction 1111.16
| 11 |
The C2H5O radical will transform into CH3CHO through electron impact or oxidative reactions, as shown in reactions 1212 and 13. CH3CHO is the most abundant byproduct of ethanol oxidation.17 O, O2, O– and O2– can all react with the C2H5O radical to produce CH3CHO, but oxidation by O2 is the main chemical pathway.16 The O2 PAES system has the highest concentration of CH3CHO compared to air PAES and N2 PAES, due to the large amount of oxygen provided, as shown in Figure 9.
| 12 |
| 13 |
In addition to the reactions mentioned above, C2H5O radicals can also combine with each other to form butanediol, as detected in Figure 9. CH3CHO can be further decomposed into CH3CO by electron impact, as shown in reaction 1414 in the gas-phase of plasma region.16 CH3CO can then react with O2 to form CH3CO3, the main precursor of CH3COOH, in reaction 1515. Besides reacting with H2O to form CH3COOH in reaction 1616, CH3CO3 can also react with HO2 to form CH3COOOH trough reaction 1717.
| 14 |
| 15 |
| 16 |
| 17 |
In addition to the reactions above that form CH3COOH and CH3COOOH, CH3CHO can be directly oxidized by O2 or O3 to form CH3COOH, as shown in reaction 1818, and by O– or O2– to form CH3COOOH, as shown in reaction 1919.18 CH3COOOH can also be produced by the oxidation of CH3COOH or the reaction between CH3COOH and H2O2, as shown in reactions 2020 and 21.18,19Reaction 2121 can occur in the liquid-phase.
| 18 |
| 19 |
| 20 |
| 21 |
It is worth noting that reactions related to aqueous electrons are not considered in this study. Due to the large number of electrons attached to oxygen in the oxygen plasma (reaction 55), the electron density is reduced. Furthermore, this leads to a decrease in the number of aqueous electrons. In addition, the aqueous electrons can also be scavenged by the ROS formed by the oxygen plasma in the liquid phase. This will result in a significant reduction of aqueous electrons.9
The reaction pathways in the O2 PAES system are visually illustrated in Figure 13.
Figure 13.
Reaction pathways in O2 PAES system.
3.6. The Applications of PAES
Indeed, plasma treatment of ethanol has been explored for various applications such as H2 production.20 In those studies, Ar and N2 are commonly used as the working gases for plasma.11 Rincón et al.21 reported that H2 was generated with up to 85% selectivity in an Ar microwave plasma-ethanol system. Burlica et al.22 investigated the formation rate and energy yield of H2 from methanol, propanol, and ethanol solutions exposed to a nonthermal pulsed plasma-gliding arc reactor, achieving the highest H2 energy yield (176 g·kW·h–1) with Ar gas and pure methanol.
However, O2 plasma is seldom employed for H2 production in plasma ethanol systems due to its tendency to produce OH through reactions with water, which can rapidly consume the H generated in reactions 77 and 8.
In contrast, our study focuses innovatively on the aqueous products when plasma reacting with ethanol solution. For the first time, we discovered that O2 plasma treatment of ethanol can produce a highly active, environmentally friendly, and safe disinfectant.
The O–O bond in the CH3COOOH has a bond energy of 159 kJ·mol–1, which can be easily activated.23 CH3COOOH can be activated by UV, ultrasound, heat, and metal ions to generate the strong oxidizing radicals: OH (E0 = 2.72 eV), CH3COO (E0 = 2.24 eV), CH3COOO (E0 = 1.6 eV) and so on.24
In this plasma-ethanol system, CH3COOOH was generated in the solution during the discharge process. Then it further generates other active organic compounds under the combination effect of plasma through the following reactions:25
| 22 |
| 23 |
| 24 |
| 25 |
Therefore, in the plasma-ethanol system, aside from electrons and reactive oxygen species (ROS) in plasma to continuously react with ethanol and produce CH3COOOH, plasma also activates CH3COOOH to generate numerous free radicals. The presence of these free radicals in PAES significantly enhances its activity.
The storage stability of peroxyacetic acid (CH3COOOH) generally depends on its concentration. Lower concentrations have shorter storage times. This is why commercially CH3COOOH typically ranges from 18% to 23% concentration. When used, it often needs to be significantly diluted, a process that is hazardous and necessitates professional guidance.
The commercially peroxyacetic acid (CH3COOOH) is corrosive and irritating to the eyes, mucous membranes, and skin. It is flammable and explosive when exposed to high heat, reducing agents, or metal ions. In addition to these dangers, it emits an extremely irritating odor. The commercially CH3COOOH is currently listed in the “List of Explosive Chemicals” and is regulated under the “Measures for the Public Security Management of Explosives Precursors.″
In contrast, preparing O2 PAES is convenient, and freshly prepared O2 PAES contains abundant reactive free radicals. It remains active when stored at −20 °C, with a CH3COOOH concentration of only about 0.0166%, which is odorless and nonirritating to touch, yet ensures high efficacy. Compared to conventional disinfectants, O2 PAES is safe, gentle, and highly efficient. As a result, O2 PAES presents a viable alternative to commercially available disinfectants.
4. Conclusion
This study addresses the current use of high-concentration (70–75%) alcohol solutions as disinfectants, which are known for their drawbacks such as flammability and strong odor. To propose a new approach, the study suggests using nanosecond pulse-driven bubble discharge in low-concentration alcohol solutions for sterilization. Research findings indicate that O2 PAES exhibits superior sterilization efficacy, with a 3 min treatment of 10% alcohol eliminating all bacteria (reducing bacterial count by 7 orders of magnitude) with an energy requirement of only 10.9 J/ml, whereas the same treatment with air PAES achieves less than a one-order reduction and O2/N2/air plasma activated water (PAW) achieved even less.
The high energy efficiency achieved can be attributed to the use of bubble discharge in liquid, facilitating thorough interaction between the plasma and the solution, along with the efficient generation of high-energy electrons enabled by nanosecond pulse driving.
Furthermore, to delve deeper into the key factors of PAES sterilization, various working gases for the plasma have been explored to modulate essential reactions. Reactive species concentrations were measured using assay kits and GC-MS, correlating them with bactericidal effects. Results indicate that using air as the working gas results in the highest NO2– + NO3– concentration, which decreases with increasing alcohol proportion, suggesting that NO2– + NO3– is not a critical bactericidal factor in PAES. pH measurements revealed that air PAES reaches its lowest pH (3 min treatment: pH 3.3, 5 min treatment: pH 3.0).
When O2 is used as the working gas in PAES, it results in the highest concentration of H2O2, which initially increases with alcohol proportion and then decreases.
Further experiments storing O2 PAES at 4 °C and −20 °C showed no significant difference in H2O2 and pH trends between conditions, yet significant differences in bactericidal efficacy were observed. At 4 °C, PAES lost efficacy within a day (less than 3 orders of magnitude reduction after 1 day, less than 1 order of magnitude reduction after 4 days), whereas at −20 °C, PAES maintained complete bacterial elimination even after 4 days, reducing bacterial count by approximately 5 orders of magnitude after 5 days. This indicates that H2O2 and pH are not crucial factors in the sterilization process.
Additionally, GC-MS analysis revealed the presence of various organic compounds in PAES, including CH3CHO, CH3CH2OH, CH3COOH, and CH3COOOH. Comparisons of these compounds among air/N2/O2 PAES and the concentration of Peroxyacetic acid (CH3COOOH) over time at different storage temperatures correlated with their bactericidal effects, identifying peroxyacetic acid as a key bactericidal factor in PAES.
In conclusion, this experiment introduces a novel, environmentally friendly, efficient, and safe method for preparing disinfectants based on plasma-activated low-concentration ethanol solutions. Moreover, the plasma device used in this study is easily scalable and adaptable to various practical needs.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (Grant No. 2021YFE0114700) and National Natural Science Foundation of China (Grant Nos. 52130701 and 52277150).
Author Contributions
# Y.Q.L. and K.S. contributed equally to this work.
The authors declare no competing financial interest.
References
- Itiki R.; Chowdhury P. R. Fast deployment of COVID-19 disinfectant from common ethanol of gas stations in Brazil. Health Policy Technol. 2020, 9 (3), 384–390. 10.1016/j.hlpt.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R. W.; Zhou R. S.; Prasad K.; Fang Z.; Speight R.; Bazaka K.; Ostrikov K. Cold atmospheric plasma activated water as a prospective disinfectant: the crucial role of peroxynitrite. Green Chem. 2018, 20 (23), 5276–5284. 10.1039/C8GC02800A. [DOI] [Google Scholar]
- Bruggeman P. J.; Bogaerts A.; Pouvesle J. M.; Robert E.; Szili E. J. Plasma–liquid interactions. J. Appl. Phys. 2021, 130 (20), 2004201. 10.1063/5.0078076. [DOI] [Google Scholar]
- Zhou R. W.; Zhang T. Q.; Zhou R. S.; Wang S.; Mei D. H.; Mai-Prochnow A.; Weerasinghe J.; Fang Z.; Ostrikov K.; Cullen P. J. Sustainable plasma-catalytic bubbles for hydrogen peroxide synthesis. Green Chem. 2021, 23 (8), 2977–2985. 10.1039/D1GC00198A. [DOI] [Google Scholar]
- Tampieri F.; Gorbanev Y.; Sardella E. Plasma-treated liquids in medicine: Let’s get chemical. Plasma Processes Polym. 2023, 20 (9), e2300077 10.1002/ppap.202300077. [DOI] [Google Scholar]
- Li Y. Q.; Liu J. L.; Zhao F.; Song K.; Nie L. L.; Liu D. W.; Lu X. P. Plasma-activated ethanol solution and it’s decontamination effect. High Volt. 2023, 8 (4), 833–840. 10.1049/hve2.12299. [DOI] [Google Scholar]
- Tao S.; Cheng Z.; Ruixue W.; Ping Y.; Chengyan R. Atmospheric-pressure Pulsed Gas Discharge and Pulsed Plasma Application. High Volt. Eng. 2016, 42 (3), 685–705. [Google Scholar]
- Su Y. Y.; Liu S.; Zhao C. F.; Yang X. J.; Huang L.; Peng X.; Liu D. Q. Needle electrode design of pulsed high voltage discharge reactor for performance enhancement of 4-chlorophenol degradation in highly conductive solution. Chemosphere 2021, 266, 129203. 10.1016/j.chemosphere.2020.129203. [DOI] [PubMed] [Google Scholar]
- Chen Q.; Lin J.; He X. Y.; Hu H. L.; Li J. S.; Xiong Q.; Chen B. Y.; Song Z. Y.; Liu H.; Liu Q. H.; Liu K. Ethanol-controlled peroxidation in liquid-anode discharges. J. Phys. D: Appl. Phys. 2019, 52 (42), 425205. 10.1088/1361-6463/ab324a. [DOI] [Google Scholar]
- Lukes P.; Dolezalova E.; Sisrova I.; Clupek M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Sci. Technol. 2014, 23 (1), 015019. 10.1088/0963-0252/23/1/015019. [DOI] [Google Scholar]
- Du C. M.; Mo J. M.; Li H. X. Renewable Hydrogen Production by Alcohols Reforming Using Plasma and Plasma-Catalytic Technologies: Challenges and Opportunities. Chem. Rev. 2015, 115 (3), 1503–1542. 10.1021/cr5003744. [DOI] [PubMed] [Google Scholar]
- Levko D.; Sharma A.; Raja L. L. Non-thermal plasma ethanol reforming in bubbles immersed in liquids. J. Phys. D: Appl. Phys. 2017, 50 (8), 085202. 10.1088/1361-6463/aa4ea0. [DOI] [Google Scholar]
- Sato T.; Furuya O.; Ikeda K.; Nakatani T. Generation and transportation mechanisms of chemically active species by dielectric barrier discharge in a tube for catheter sterilization. Plasma Processes Polym. 2008, 5 (6), 606–614. 10.1002/ppap.200800035. [DOI] [Google Scholar]
- Shen J.; Zhang H.; Xu Z. M.; Zhang Z. L.; Cheng C.; Ni G. H.; Lan Y.; Meng Y. D.; Xia W. D.; Chu P. K. Preferential production of reactive species and bactericidal efficacy of gas liquid plasma discharge. Chem. Eng. J. 2019, 362, 402–412. 10.1016/j.cej.2019.01.018. [DOI] [Google Scholar]
- Uchiyama H.; Ishikawa K.; Zhao Q. L.; Andocs G.; Nojima N.; Takeda K.; Krishna M. C.; Ishijima T.; Matsuya Y.; Hori M.; Noguchi K.; Kondo T. Free radical generation by non-equilibrium atmospheric pressure plasma in alcohol–water mixtures: an EPR-spin trapping study. J. Phys. D: Appl. Phys. 2018, 51 (9), 095202. 10.1088/1361-6463/aaa885. [DOI] [Google Scholar]
- Lovascio S.; Blin-Simiand N.; Magne L.; Jorand F.; Pasquiers S. Experimental Study and Kinetic Modeling for Ethanol Treatment by Air Dielectric Barrier Discharges. Plasma Chem. Plasma Process. 2015, 35 (2), 279–301. 10.1007/s11090-014-9601-x. [DOI] [Google Scholar]
- Wang W. J.; Zhu C. Y.; Cao Y. Y. DFT study on pathways of steam reforming of ethanol under cold plasma conditions for hydrogen generation. Int. J. Hydrogen Energy 2010, 35 (5), 1951–1956. 10.1016/j.ijhydene.2009.12.170. [DOI] [Google Scholar]
- Xia W. J.; Liu D. X.; Guo L.; Wang W. T.; Xu H.; Feng C.; Wang X. H.; Kong M. G.; Rong M. Z. Discharge characteristics and bactericidal mechanism of Ar plasma jet with ethanol and oxygen gas admixtures. Plasma Sources Sci. Technol. 2019, 28 (12), 125005. 10.1088/1361-6595/ab5168. [DOI] [Google Scholar]
- Zhao X. B.; Zhang T.; Zhou Y. J.; Liu D. H. Preparation of peracetic acid from hydrogen peroxide-Part 1: kinetics for peracetic acid synthesis and hydrolysis. J. Mol. Catal. A: Chem. 2007, 271 (1–2), 246–252. 10.1016/j.molcata.2007.03.012. [DOI] [Google Scholar]
- Aubry O.; Met C.; Khacef A.; Cormier J. M. On the use of a non-thermal plasma reactor for ethanol steam reforming. Chem. Eng. J. 2005, 106 (3), 241–247. 10.1016/j.cej.2004.12.003. [DOI] [Google Scholar]
- Rincón R.; Marinas A.; Muñoz J.; Melero C.; Calzada M. D. Experimental research on ethanol-chemistry decomposition routes in a microwave plasma torch for hydrogen production. Chem. Eng. J. 2016, 284, 1117–1126. 10.1016/j.cej.2015.09.062. [DOI] [Google Scholar]
- Burlica R.; Shih K. Y.; Hnatiuc B.; Locke B. R. Hydrogen Generation by Pulsed Gliding Arc Discharge Plasma with Sprays of Alcohol Solutions, Ind. Eng. Chem. Res. 2011, 50 (15), 9466–9470. 10.1021/ie101920n. [DOI] [Google Scholar]
- Xie P. C.; Guo Y. Z.; Chen Y. Q.; Wang Z. P.; Shang R.; Wang S. L.; Ding J. Q.; Wan Y.; Jiang W.; Ma J. Application of a novel advanced oxidation process using sulfite and zero-valent iron in treatment of organic pollutants. Chem. Eng. J. 2017, 314, 240–248. 10.1016/j.cej.2016.12.094. [DOI] [Google Scholar]
- Luukkonen T.; Pehkonen S. O. Peracids in water treatment: A critical review. Crit. Rev. Environ. Sci. Technol. 2017, 47 (1), 1–39. 10.1080/10643389.2016.1272343. [DOI] [Google Scholar]
- Liu Y.; Li D. R.; Chen M. N.; Sun Q. Y.; Zhang Y.; Zhou J.; Wang T. C. Radical adducts formation mechanism of CH3CO2• and CH3CO3• realized decomposition of chitosan by plasma catalyzed peracetic acid. Carbohydr. Polym. 2023, 318, 121121. 10.1016/j.carbpol.2023.121121. [DOI] [PubMed] [Google Scholar]