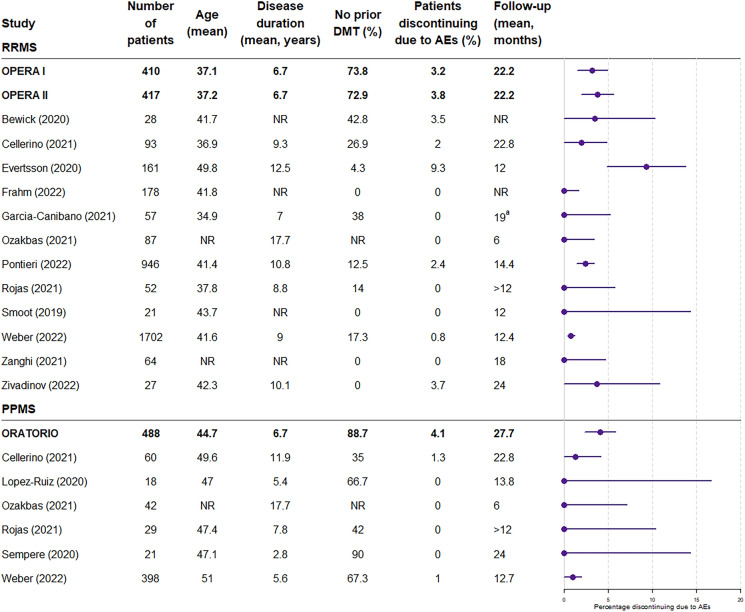
Fig. 2.
Patients discontinuing due to adverse events: clinical trials versus real-world studies. 95% binomial confidence intervals were calculated for the proportion of patients discontinuing due to adverse events, and the rule of three was used in the case of zero counts. Note that the rule of three is a very good approximation when n > 30, and is a good approximation, although slightly less accurate, when n < 30. AE adverse event, DMT disease-modifying therapy, PPMS primary progressive multiple sclerosis, RRMS relapsing remitting multiple sclerosis