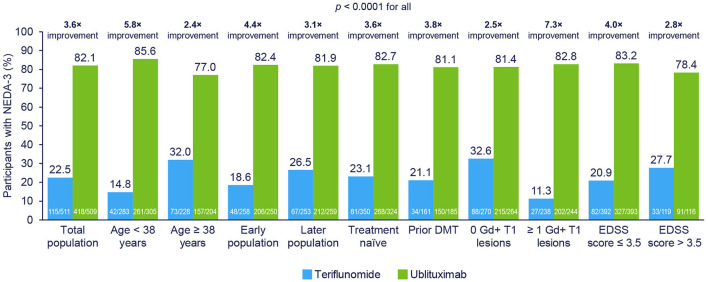
Figure 4.
NEDA-3 at Weeks 24–96 (re-baselined) in participant subgroups. NEDA-3 was defined as no confirmed relapses, no Gd+ T1 lesions, no new or enlarging T2 lesions, and no 12-week confirmed disability progression. Early disease population vs. later disease population, defined as <or ≥ median time, was approximately 3 years from MS diagnosis to study randomization. Pooled post hoc analysis. Modified intention-to-treat population. DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; Gd+, gadolinium-enhancing; NEDA-3, 3-parameter no evidence of disease activity.