Abstract
Background
Ki67 index changes during the treatment of metastatic pancreatic neuroendocrine tumor (PanNET) treatment. The study aimed to detect alterations of grade based on Ki67 index and immune microenvironment in PanNET responding to capecitabine/temozolomide (CapTem).
Method
Retrospective data of patients with PanNET were collected. In control group, 35 patients underwent surgery immediately after biopsy. In CapTem group, 38 patients received CapTem after biopsy and responded well to treatment (defined as either stable disease or partial response), and subsequently underwent surgery. All patients have pathological Ki67 index at biopsy and after surgery. CD163 + CD68 + CD206 + M2 macrophages, CD68 + CD86 + CD80 + M1 macrophages, CD11b + CD33 + myeloid-derived suppressor cells, and CD4 + CD25 + regulatory T cells were stained using multiplex immunofluorescence.
Results
In control group, the paired grade based on Ki67 index directly after surgery showed no upgrade or downgrade compared to biopsy. In patients who responded well to CapTem, the grade based on Ki67 index before and after CapTem was altered. Thirteen patients had upgraded Ki67 index and 11 patients had downgraded. The proportion of stable disease was higher in the upgraded group compared to downgraded group (p = 0.0155). And upgraded group had a significantly shorter mPFS than patients in the downgrade group (8.5 months vs. 20 months, HR 4.834, 95% CI 1.414 to 16.53, p = 0.012). M1 macrophages was significantly lower in the downgraded group than in the Ki67 upgraded group (p < 0.001).
Conclusion
Grade based on Ki67 index and immune environment change in PanNET patients responding well to CapTem. Patients with downgraded had longer mPFS compared to those with upgraded. It is necessary to reassess the Ki67 index after CapTem treatment, even in patients responding well to CapTem.
Keywords: Pancreatic neuroendocrine tumor, Ki67 index, Capecitabine/temozolomide, Upgrading, Tumor microenvironment
Introduction
Pancreatic neuroendocrine neoplasms (PanNENs) represent a rare and heterogeneous entity, accounting for 3–5% of all pancreatic neoplasms, including well-differentiated pancreatic neuroendocrine tumors (PanNETs) and poor-differentiated pancreatic neuroendocrine carcinomas [1]. Ki67 index serves as a crucial marker for both the diagnosis and prognosis of PanNENs [2–4]. According to the Ki67 index and mitosis, well-differentiated PanNETs have been subdivided into three groups (G1, G2 and G3) [5], with 5-year survival rates of 75%, 62%, and 7%, respectively [1].
Grade based on Ki67 index exhibits variability, not only among different stages of PanNETs, but also between the primary site and metastatic sites, showing a statistically significant increase in Ki67 index, which lead to upgraded, in metastases sites compared to the primary lesion [6, 7]. Additionally, the effectiveness of systemic therapies influences the grade based on Ki67 index. The standard treatment for metastatic PanNETs includes various systemic therapies to effectively address disease progression, such as Somatostatin analogues (SSA), sunitinib, and capecitabine/temozolomide (CapTem) [8–11]. Studies have noted that a majority of cases experienced an increase in Ki67 index leading to an upgrade to a higher WHO grade as the disease progressed [12, 13]. Moreover, upgrading by increasing Ki67 index during disease progression predicts an unfavorable outcome [14]. Therefore, clinicians primarily concentrate on monitoring Ki67 alterations during disease progression. Nevertheless, data regarding the variability in Ki67 index and its prognostic implications in case of successful treatment for PanNET cases is still scanty.
We present a cohort of patients with metastatic PanNETs who exhibited a positive response to chemotherapy (defined as stable disease or partial response to CapTem) followed by surgical intervention. We conducted an anlysis of the changes in grade based on Ki67 index and the immune microenvironment pre- and post-CapTem treatment.
Methods and materials
Patients’ selection and exclusion criteria
Retrospective real-world data of PanNET cases treated between September 2003 and January 2023 at the Fudan university Shanghai Cancer Center (FUSCC) were collected. GHL and ZWH conducted a blind calculation of Ki67 index from original slides. The selection criteria included: (1) Histologically confirmed well-differentiated NETs; (2) Primary tumor located in the pancreas; (3) Presence of liver metastasis; (4) Non-functional tumors; (5) CapTem treatment with stable disease or partial response assessed by RECIST 1.1; (6) Direct surgery or post-CapTem surgery; (7) Availability of pathological ki67 index staining pre- and post-treatment; (8) Consistent 18 F-FDG uptake between the primary site and corresponding liver metastasis. The exclusion criteria comprised: (1) Pathological report indicating mixed neuroendocrine non-neuroendocrine neoplasm; (2) Presence of genetic syndromes, such as multiple endocrine neoplasia (MEN) or von Hippel-Lindau syndrome (VHL); (3) Inadequate tissue lacking a precise Ki67 index; (4) Previous receipt of multiple systemic therapies pre-surgery or treatment like radiofrequency ablation or transcatheter arterial embolization.
Patients characteristics Collection
A total of 73 PanNET patients were included in the study. Patient characteristics, including demographics (age and sex), pathological reports (T stage, N stage, MGMT IHC situation, tumor grade, and Ki67 index at each time point), and treatment details (treatment duration, response assessment, surgery), were collected. Tumor staging was based on the 2010 European Neuroendocrine Tumor Society (ENETS) TNM staging system for PanNET patients [15]. Tumor grading and differentiation adhered to the 2019 WHO classification [5]. Downgrading defined as change in the Ki67 index led to downgrading from NET G2 to G1, or G3 to G2/G1; and upgrading defined as change in the Ki67 index led to upgrading from NET G1 to G2/3, or G2 to G3. Patients were followed up through outpatient visits until August 2023. For patients who underwent surgery following successful chemotherapy, the duration of treatment before surgery was recorded.
Patients underwent follow-up at 3-6-month intervals using enhanced computed tomography or magnetic resonance imaging. Tumor response to systemic therapy was defined as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) according to the Response Evaluation Criteria for Solid Tumors (RECIST), version 1.1 [16]. The Multi-Disciplinary Treatment group evaluated the tumor response to therapies and decided the possibility of resection. Progression-free survival (PFS) defined as the time between post-surgery and the disease progression.
Multispectral fluorescent IHC
Formalin-fixed paraffin-embedded (FFPE) slides from specimens were subjected to multispectral immunohistochemical (mIHC) staining using the Opal color kit (PerkinElmer, Hopkinton, Massachusetts, USA) according to the manufacturer’s instructions. Primary antibodies included the following markers: CD68 (GB113150, 1:1000, Servicebio, Wuhan, China), CD80 (305202, 1:3000, BioLegend, San Diego, USA), CD86 (ab269587, 1:100, Abcam, Cambridge, UK), CD163 (16646-1-AP, 1:200, Proteintech, Wuhan, China), CD206 (CST-91992 S, 1:200, Cell Signaling, Danvers, USA), CD11b (21851-1-AP, 1:500, Proteintech, Wuhan, China), CD33 (17425-1-AP, 1:25, Proteintech, Wuhan, China), CD4 (A19018, 1:100, Abclonal, Wuhan, China), and CD25 (ABB109, 1:200, Abbrab, Shanghai, China). The definition of staining cell types within the adaptive immune system, including the M1-like phenotype (CD68 + CD80 + CD86+), M2-like phenotype (CD68 + CD163 + CD206+), myeloid-derived suppressor cells (MDSC, CD11b + CD33+), and Treg (CD4 + CD25+). Cell nuclei were stained with DAPI. Primary antibodies were sequentially applied, followed by horseradish peroxidase-conjugated secondary antibody incubation (1:1, DS9800, Leica Biosystems, Shanghai, China; 1:1 Cat# A10011-6/A10012-6, WiSee Biotechnology, Beijing, China), and tyramide signal amplification (M-D110051, WiSee Biotechnology, Beijing, China). The slides were microwave heat-treated after each TSA operation. The stained slides were scanned to obtain multispectral images using the PerkinElmer Vectra automated multispectral microscope at 100× magnification and analyzed using PerkinElmer inForm Analysis Software.
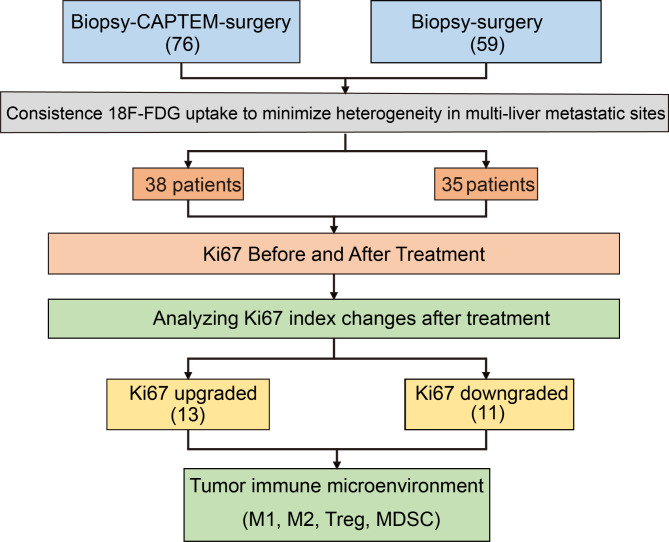
Fig. 1.
Flow diagram for study
Statistical analyses
The correlation between the clinicopathological and therapeutic parameters specified by the classification system was analyzed. Qualitative variables were analyzed using the χ2 test or Fisher’s exact test. Quantitative variables were analyzed either by analysis of variance (ANOVA) or the non-parametric Kruskal-Wallis test, depending on whether the results followed a normal distribution or not. Kaplan-Meier curves depicting the time to progressive disease were computed using the log-rank test to verify the significance of differences between survival curves. Univariate and multivariate Cox proportional models were used to investigate the effects of several prognostic factors on the risk of recurrence. Hazard ratios (HRs) and confidence intervals (CIs) were also estimated using the Cox model. Statistically significant factors (P < 0.05) identified by univariate analysis were included in the multivariate analysis. Statistical tests were performed using Prism 8, version 8.4.0 (455). Statistical significance was set at p < 0.05, and all tests were two-sided.
Results
Patients characteristic
Treatment strategies and the screening process are shown in Fig. 1. Finally, 73 patients with well-differentiated non-functional liver metastatic PanNETs were included. Table 1 summarizes the clinicopathological features of the patients. Thirty-eight patients received CapTem and responded well to the treatment, which was defined as either stable disease or partial response, then underwent surgery. Thirty-five patients underwent surgery immediately after the biopsy. There were no differences in baseline characteristics, such as age, sex, grade, T stage, and N stage.
Table 1.
Patients characteristic
| CapTem – Surgery (38 patients) |
Surgery (35 patients) |
P value | |
|---|---|---|---|
| Age median (range) | 52(29–68) | 48(18–73) | 0.837 |
| Gender | |||
| Female | 18 (47.4%) | 17 (48.6%) | 0.891 |
| Male | 20 (52.6%) | 18 (51.4%) | |
| Location | |||
| Head/Neck | 10 (26.3%) | 11 (31.4%) | 0.823 |
| Body/Tail | 28 (73.7%) | 24 (68.6%) | |
| T stage | |||
| T1 | 5 (13.2%) | 4 (11.5%) | 0.895 |
| T2 | 14 (36.8%) | 15 (42.8%) | |
| T3 | 13 (34.2%) | 13 (37.1%) | |
| T4 | 6 (15.8%) | 3 (8.6%) | |
| N stage | |||
| N0 | 16 (42.1%) | 12 (34.3%) | 0.655 |
| N1 | 22 (57.9%) | 23 (65.7%) | |
| Ki67 before treatment | |||
| G1 | 6 (15.8%) | 9 (25.7%) | 0.602 |
| G2 | 25 (65.8%) | 23 (65.7%) | |
| G3 | 7 (18.4%) | 3 (8.6%) | |
| Ki67 after surgery | |||
| G1 | 7 (18.4%) | 9 (25.7%) | 0.829 |
| G2 | 24 (63.2%) | 23 (65.7%) | |
| G3 | 7 (18.4%) | 3 (8.6%) |
CapTem: capecitabine combined with temozolomide; SSA: Somatostatin analogues;
Ki67 alteration between biopsy and surgery
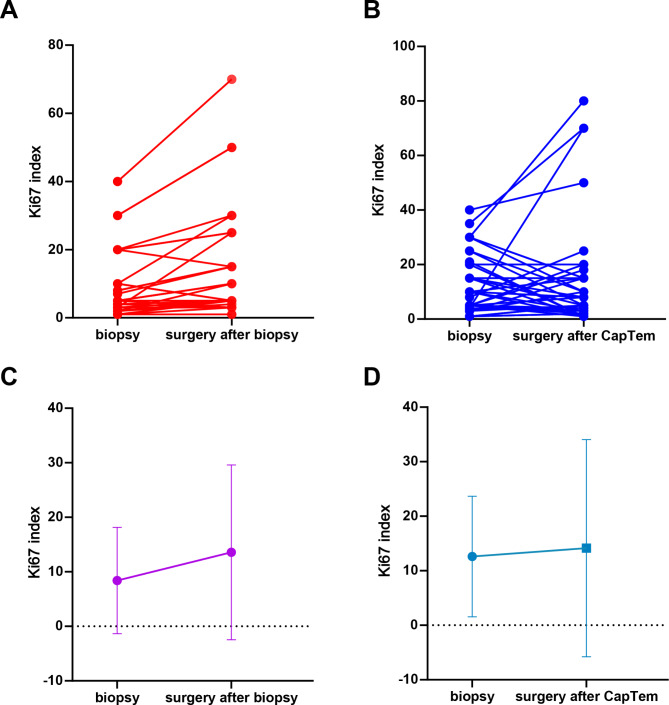
First, grade based on Ki67 alterations between the biopsy and surgery were detected. Thirty-five patients received resection of the primary site and liver metastasis directly after liver biopsy. The median time from biopsy to surgery was one month. Compared to Ki67 index by biopsy, the paired grade based on Ki67 index after surgery kept consistency with no upgrade or downgrade (p = 0.103, Fig. 2A, C).
Fig. 2.
Paired Ki67 index by biopsy and surgery
(A, C) Ki67 index alteration in biopsy-surgery group (A) paired Ki67 index for each patient, (C) median Ki67 index. (B, D) Ki67 index alteration in biopsy-CapTem-surgery group (B) paired Ki67 index for each patient, (D) median Ki67 index
Thirty-eight patients with PanNETs received CapTem chemotherapy after undergoing biopsy. The median duration of CapTem treatment was 9 cycles (range: 3 to 20 cycles). The paired grade based on Ki67 index of the biopsy (before CapTem) and surgery (after CapTem) were changed (p = 0.0012, Fig. 2B, D). After Ki67 index evaluation, 13 patients were re-classified as upgraded (from G1 to G2, or G2 to G3). While changes in the Ki67 index led to downgrading in 11 patients (from G3 to G2/G1, or G2 to G1). The remaining 14 patients showed no changes in grade.
Ki67 index alteration after chemotherapy associated with PanNET prognosis
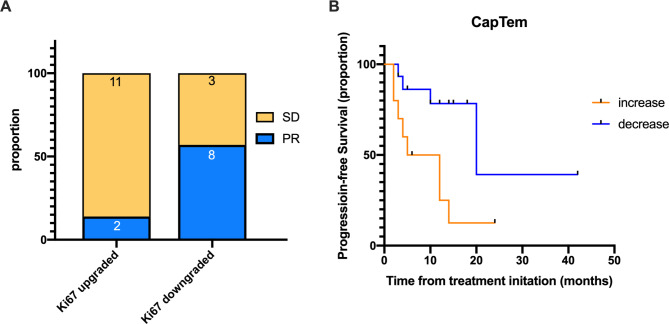
In the CapTem treatment group, fourteen patients had PR and 24 patients had SD. In the upgraded group, the proportions of patients with PR and SD were 15.4% (2/13) and 84.6% (11/13), respectively. While in the downgraded group, PR and SD were72.7% (8/11) and 27.3% (3/11), respectively. The proportion of SD was higher in the upgraded group (p = 0.0155, Fig. 3A). The median progression-free survival (mPFS) after surgery showed that patients in the upgraded group had a significantly shorter mPFS compared to patients in the downgrade group (8.5 months vs. 20 months, HR 4.834, 95% CI 1.414 to 16.53, p = 0.012, Fig. 3B). Low expression of MGMT showed no difference between the upgraded or downgraded group.
Fig. 3.
ORR and mPFS in Ki67 upgraded and Ki67 downgraded group after CapTem treatment
(A) PR and SD rate in Ki67 upgraded group (13 patients) and Ki67 downgraded group (11 patients). (A, B) mPFS in Ki67 upgraded and Ki67 downgraded group
ORR: objective response rate; mPFS: median progression-free survival; PR: partial response; SD: stable disease
The immune environment changes after CapTem treatment
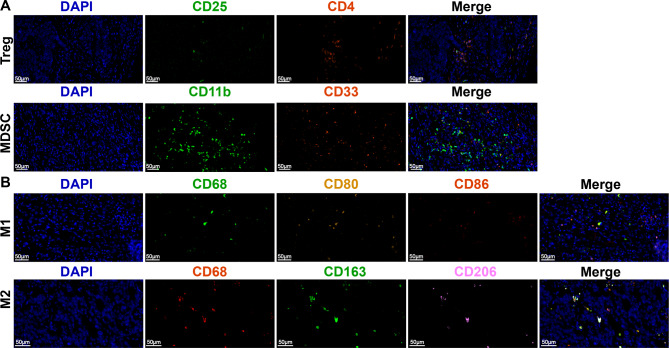
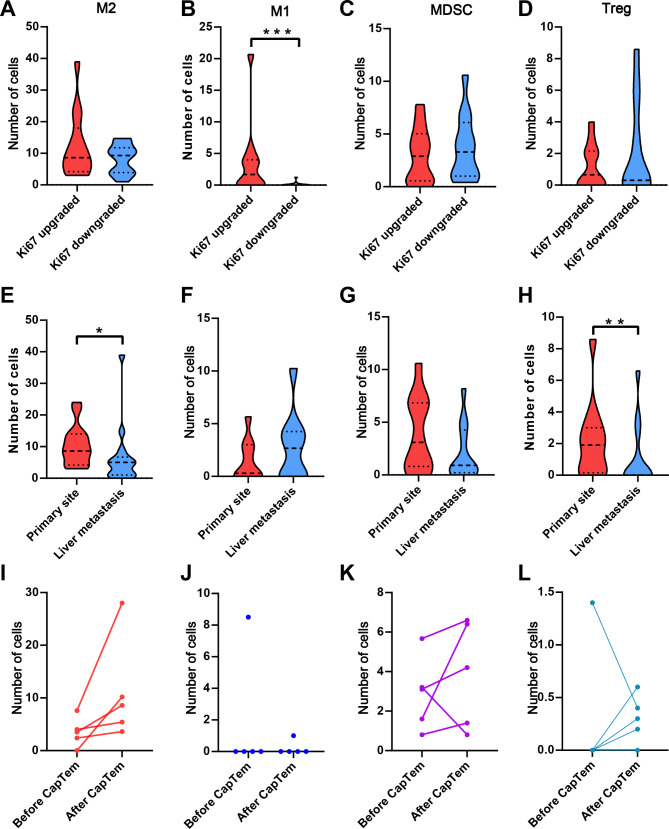
Given the influence of TMZ on the tumor immune environment, we conducted a detailed analysis of immune environment changes in the upgraded and downgraded groups following CapTem treatment. Immunohistochemistry staining was performed on M2 cells (CD163 + CD68 + CD206+), M1 cells (CD68 + CD86 + CD80+), MDSCs (CD11b + CD33+) and Treg (CD4 + CD25+) using multispectral fluorescent IHC (Fig. 4). The results showed a significantly higher number of peritumoral infiltrating immune cells compared to intratumoral counterparts (p < 0.001 for each). Compared to the upgraded group, the average number of M1 cells was markedly reduced in the downgraded group (3.569 ± 3.15 vs. 0.131 ± 7.67, p < 0.001). However, there were no discernible differences in M2 cells, MDSCs, and Treg between the two groups (Fig. 5A-D). Comparison of paired primary and liver metastatic sites revealed a higher presence of Treg and M2 cells in the liver metastatic site than in the primary site, with no significant disparity in MDSCs and M1 cells (Fig. 5E-H).
Fig. 4.
Image of multispectral fluorescent IHC staining
(A) CD163 + CD68 + CD206+; (B) CD68 + CD86 + CD80+, (C) CD11b + CD33+, (D) CD4 + CD25+
IHC: immunohistochemical
Fig. 5.
Comparing immune cells in different groups
(A-D) Comparing M2, M1, MDSC, and Treg cells in Ki67 upgraded group and Ki67 downgraded group, respectively. (E-H) Comparing M2, M1, MDSC, and Treg cells in paired primary site and liver metastatic sites after CapTem treatment, respectively. (I-L) Comparing M2, M1, MDSC, and Treg cells in paired tissues before and after CapTem treatment. *** p < 0.001, ** p < 0.01
M2: macrophage M2-like; M1: macrophage M1-like; MDSC: myeloid-derived suppressor cells
Five patients had paired tissue samples taken before and after CapTem treatment, with four in the downgraded group and one in upgraded group. MDSCs and M2 cells was higher after CapTem treatment compared to the paired tissue before CapTem, while Treg and M1 cells remained consistently low before and after CapTem treatment (Fig. 5I-L).
Discussion
Heterogeneity of grade based on Ki67 index in PanNETs is previously mainly focused on the group of patients with disease progression [8, 10]. However, there is a gap in research regarding Ki67 index alterations in patients who respond well to chemotherapy. Our study showed that grading change based on Ki67 index after CapTem treatment in responsive patients significantly associated with both survival outcomes and the tumor immune microenvironment. Notably, patients with downgrading exhibited a longer mPFS compared to those with upgrading. It is crucial to closely monitor changes following CapTem treatment, particularly in patients showing positive responses.
The potential bias in Ki67 index heterogeneity may raise concerns among researchers. Our study was designed proactively to address this issue. To mitigate bias, we specifically included patients without heterogeneity in 18 F-FDG uptake at metastatic sites, considering the variability of Ki67 index between primary and metastatic sites. 18 F-FDG uptake could reflect the pathological grade of PanNET lesions, enabling the assessment of tumor biological aggressiveness and guiding the selection of the most relevant lesions for biopsy [17]. No significant differences in 68Ga-DOTANOC SUVmax were observed with changes in the Ki67 index [18]. Another source of Ki67 heterogeneity arises from differences in biopsy and surgery approaches. Grade of PanNETs by liver biopsy and fine needle aspiration (FNA) is acceptable [19]. The intra-class correlation of Ki67 index between pre-surgical biopsies and surgical specimens was 0.99, and Ki67 classes were accurately identified in 97% of the biopsies [20]. In our study, we established biopsy-surgery group as control, revealing consistent grade based on Ki67 index values within this group.
In biopsy-CapTem-surgery group, however, grade based on Ki67 index after CapTem treatment exhibited variability, with some cases upgraded while others downgraded. Chemotherapy influenced Ki67 index. Cases series reported an increase in 68Ga-DOTATATE PET-CT avidity after treatment with CapTem [21]. Temozolomide (TMZ) reduced 18 F-FFT-PET uptake significantly, accompanied by a significant decrease in tumor volume in glioma [22]. Our results showed that in patients respond well to CapTem, grade alteration based on Ki67 index associated with prognosis of PanNET patients, with those experiencing upgraded showing a shorter mPFS compared to those with downgraded.
Subsequently, we conducted an in-depth analysis of the immune microenvironment in PanNET patients responsing well to CapTem treatment, focusing on changes in macrophages (M1 and M2 phenotypes), Treg and MDSC post-treatment. Reports showed that 32–65% of PanNETs had high T-cell infiltrates in intratumoral area, with 50–70% showing high levels of T memory and cytotoxic T-cells infiltration in extratumoral compartment [23]. Chemotherapy plays a pivotal role in shaping the tumor immune environment, as evidenced by studies demonstrating that TMZ leads to a reduction in CD4 + and CD8 + T cells, an increase in Treg and MDSCs [24, 25], and the potential loss of mismatch repair (MMR) gene function, potentially sensitizing tumors to immune checkpoint inhibitor [26, 27]. Low-dose TMZ before dendritic cell vaccination reduces Foxp3 + Treg cells in advanced melanoma patients [28]. Macrophages infiltration in PanNETs might correlate with tumor progression and metastatic behavior [29]. Macrophages infiltration in the tumor tissue can be used as a biomarker for chemotherapy, as increased infiltration of macrophages reduced chemotherapy efficacy in colorectal cancer [30]. In our study, we found that the number of M1-like cells differed in different Ki67 index group, with a lower count in the downgraded group compared to the upgraded group. There were no differences in M2, Treg, and MDSC counts between the upgraded and downgraded groups. Further investigations are necessary to assess the effects of M1 cells following CapTem treatment.
Our study has several limits indeed. Firstly, the retrospective nature of the current series inherently limits its scope. Every patient in our clinical management group received standard treatment and follow-up examinations strictly, these measures can cover the limits to some extent. Secondly, affected by the technique, we did not have enough paired biopsy tissue by FNA for multispectral fluorescent IHC. The paired IHC before and after CapTem were insufficient for robust statistical analysis. Since we only had five paired tissues before and after CapTem treatment, we were unable to analyze the relationship between Ki67 alteration and changes in the immune environment during CapTem treatment. The third limitation is that post-CapTem progression treatment was not tracked, warranting further exploration of the relationship between immune cells alteration and immunotherapy. Further prospective studies will be designed to acquire paired tissues before and after chemotherapy to comprehensively analyze the immune environment.
In conclusion, grade alteration based on Ki67 index were observed in PanNET patients who responded well to CapTem, with both upgrades and downgrades noted. Patients with downgraded have a longer mPFS compared to those with upgraded. Additionally, the upgraded group showed a higher presence of M1-like macrophages compared to the downgraded group. Clinicians should pay attention to changes in Ki67 index following CapTem treatment, particularly in patients who responding well to CapTem.
Acknowledgements
Not applicable.
Abbreviations
- ANOVA
Analysis of variance
- CapTem
Capecitabine plus temozolomide
- CIs
Confidence intervals
- CR
Complete response
- FFPE
Formalin-fixed paraffin-embedded
- FNA
Fine needle aspiration
- FUSCC
Fudan university Shanghai Cancer Center
- HRs
Hazard ratios
- MDSC
Myeloid-derived suppressor cells
- MEN
Multiple endocrine neoplasia
- mIHC
Multispectral immunohistochemical
- MMR
Mismatch repair
- mPFS
Median progression-free survival
- PanNEN
Pancreatic neuroendocrine neoplasm
- PanNETs
Pancreatic neuroendocrine tumors
- PD
Progressive disease
- PFS
Progression-free survival
- PR
Partial response
- RECIST
Response Evaluation Criteria for Solid Tumors
- SD
Stable disease
- SSA
Somatostatin analogues
- Treg
Regulatory T cells
- VHL
Von Hippel-Lindau syndrome
Author contributions
Drs. HL G, XW X, XJ Y, and SR J had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. HL G, WH Z, XW X, XJ Y, and SR J concepted and designed the study. All authors contributed to the acquisition, analysis, or interpretation of data. HL G, WH Z, XW X, XJ Y, and SR J drafted the manuscript. HL G, WH Z, Z L, and WS L performed statistical analysis. MQ L, QF Z, YH S, WY X, CJ Z, YQ, and JX, and JC provided administrative, technical, or material support. And JC, XW X, XJ Y, and SR J supervised the study. All authors read and approved the final manuscript.
Funding
National Natural Science Foundation of China (No. 82141129, 82141104,82303943).Shanghai Pujiang Program (22PJ1401800). Sailing Project of Science and Technology Commission of Shanghai Municipality ( 23YF1406900), China Postdoctoral Science Foundation (2023M730670), National Natural Science Foundation of China (U21A20374), Shanghai Municipal Science and Technology Major Project (21JC1401500), Scientific Innovation Project of Shanghai Education Committee (2019-01-07-00-07-E00057), Clinical Research Plan of Shanghai Hospital Development Center (SHDC2020CR1006A), and Xuhui District Artificial Intelligence Medical Hospital Cooperation Project (2021-011).
Data availability
All data generated or analyzed during this study are included in this article.
Declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of Fudan university Shanghai Cancer Center (FUSCC, 1907204-19-1908) in 2022, and written informed consent was obtained for the utilization of patient information in research.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Heli Gao, Wuhu Zhang and Zheng Li contributed equally to this work and share first authorship
Contributor Information
Xianjun Yu, Email: yuxianjun@fudanpci.org.
Xiaowu Xu, Email: xuxiaowu@fudanpci.org.
Shunrong Ji, Email: jishunrong@fudanpci.org.
References
- 1.Cives M, Strosberg JR. Gastroenteropancreatic neuroendocrine tumors. Cancer J Clin. 2018;68(6):471–87. [DOI] [PubMed] [Google Scholar]
- 2.Hill JS, McPhee JT, McDade TP, Zhou Z, Sullivan ME, Whalen GF, et al. Pancreatic neuroendocrine tumors: the impact of surgical resection on survival. Cancer. 2009;115(4):741–51. [DOI] [PubMed] [Google Scholar]
- 3.Kloppel G, Couvelard A, Perren A, Komminoth P, McNicol AM, Nilsson O, et al. ENETS Consensus guidelines for the standards of Care in Neuroendocrine tumors: towards a standardized approach to the diagnosis of gastroenteropancreatic neuroendocrine tumors and their prognostic stratification. Neuroendocrinology. 2009;90(2):162–6. [DOI] [PubMed] [Google Scholar]
- 4.Gao H, Liu L, Wang W, Xu H, Jin K, Wu C, et al. Novel recurrence risk stratification of resected pancreatic neuroendocrine tumor. Cancer Lett. 2018;412:188–93. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd RVOR, Klöppel G. J R. WHO Classification of Tumours of Endocrine Organs. Fifth Edition. IARC WHO Classification of Tumours, No 10. 2019.
- 6.Shi H, Jiang C, Zhang Q, Qi C, Yao H, Lin R. Clinicopathological heterogeneity between primary and metastatic sites of gastroenteropancreatic neuroendocrine neoplasm. Diagn Pathol. 2020;15(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furukawa T, Ozaka M, Takamatsu M, Takazawa Y, Inamura K, Inoue Y, et al. Ki-67 labeling index variability between surgically resected primary and metastatic hepatic lesions of gastroenteropancreatic neuroendocrine neoplasms. Int J Surg Pathol. 2021;29(5):475–81. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Carbonero R, Rinke A, Valle JW, Fazio N, Caplin M, Gorbounova V, et al. ENETS Consensus guidelines for the standards of Care in Neuroendocrine neoplasms. Systemic Therapy 2: Chemother Neuroendocrinol. 2017;105(3):281–94. [DOI] [PubMed] [Google Scholar]
- 9.Hicks RJ, Kwekkeboom DJ, Krenning E, Bodei L, Grozinsky-Glasberg S, Arnold R, et al. ENETS Consensus guidelines for the standards of Care in Neuroendocrine Neoplasia: peptide receptor radionuclide therapy with Radiolabeled Somatostatin Analogues. Neuroendocrinology. 2017;105(3):295–309. [DOI] [PubMed] [Google Scholar]
- 10.Caplin ME, Pavel M, Cwikla JB, Phan AT, Raderer M, Sedlackova E, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371(3):224–33. [DOI] [PubMed] [Google Scholar]
- 11.Pavel M, Valle JW, Eriksson B, Rinke A, Caplin M, Chen J, et al. ENETS Consensus guidelines for the standards of Care in Neuroendocrine neoplasms: systemic therapy - biotherapy and Novel targeted agents. Neuroendocrinology. 2017;105(3):266–80. [DOI] [PubMed] [Google Scholar]
- 12.Singh S, Hallet J, Rowsell C, Law CH. Variability of Ki67 labeling index in multiple neuroendocrine tumors specimens over the course of the disease. Eur J Surg Oncol. 2014;40(11):1517–22. [DOI] [PubMed] [Google Scholar]
- 13.Shi H, Zhang Q, Han C, Zhen D, Lin R. Variability of the Ki-67 proliferation index in gastroenteropancreatic neuroendocrine neoplasms - a single-center retrospective study. BMC Endocr Disord. 2018;18(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexandraki KI, Kaltsatou M, Kyriakopoulos G, Mavroeidi V, Kostopoulou A, Atlan K, et al. Distinctive features of pancreatic neuroendocrine neoplasms exhibiting an increment in proliferative activity during the course of the disease. Endocrine. 2021;72(1):279–86. [DOI] [PubMed] [Google Scholar]
- 15.Rindi G. The ENETS guidelines: the new TNM classification system. Tumori. 2010;96(5):806–9. [DOI] [PubMed] [Google Scholar]
- 16.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16. [DOI] [PubMed] [Google Scholar]
- 17.de Mestier L, Armani M, Cros J, Hentic O, Rebours V, Cadiot G, et al. Lesion-by-lesion correlation between uptake at FDG PET and the Ki67 proliferation index in resected pancreatic neuroendocrine tumors. Dig Liver Dis. 2019;51(12):1720–4. [DOI] [PubMed] [Google Scholar]
- 18.Ambrosini V, Campana D, Polverari G, Peterle C, Diodato S, Ricci C, et al. Prognostic value of 68Ga-DOTANOC PET/CT SUVmax in patients with neuroendocrine tumors of the pancreas. J Nucl Med. 2015;56(12):1843–8. [DOI] [PubMed] [Google Scholar]
- 19.Laskiewicz L, Jamshed S, Gong Y, Ainechi S, LaFemina J, Wang X. The diagnostic value of FNA biopsy in grading pancreatic neuroendocrine tumors. Cancer Cytopathol. 2018;126(3):170–8. [DOI] [PubMed] [Google Scholar]
- 20.Milione M, Maisonneuve P, Pellegrinelli A, Spaggiari P, Centonze G, Coppa J, et al. Ki-67 and presence of liver metastases identify different progression-risk classes in pancreatic neuroendocrine neoplasms (pNEN) undergoing resection. Eur J Surg Oncol. 2019;45(5):755–60. [DOI] [PubMed] [Google Scholar]
- 21.Basu S, Ostwal V. Observation on enhanced avidity on somatostatin receptor targeted 68Ga-DOTATATE PET-CT following therapy with everolimus and capecitabine-temozolamide: is redifferentiation akin phenomenon a reality in neuroendocrine tumors? Nucl Med Commun. 2016;37(6):669–71. [DOI] [PubMed] [Google Scholar]
- 22.Foray C, Valtorta S, Barca C, Winkeler A, Roll W, Muther M, et al. Imaging temozolomide-induced changes in the myeloid glioma microenvironment. Theranostics. 2021;11(5):2020–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.da Silva A, Bowden M, Zhang S, Masugi Y, Thorner AR, Herbert ZT, et al. Characterization of the neuroendocrine tumor Immune Microenvironment. Pancreas. 2018;47(9):1123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karachi A, Yang C, Dastmalchi F, Sayour EJ, Huang J, Azari H, et al. Modulation of temozolomide dose differentially affects T-cell response to immune checkpoint inhibition. Neuro Oncol. 2019;21(6):730–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Germano G, Lamba S, Rospo G, Barault L, Magri A, Maione F, et al. Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth. Nature. 2017;552(7683):116–20. [DOI] [PubMed] [Google Scholar]
- 26.Daniel P, Meehan B, Sabri S, Jamali F, Sarkaria JN, Choi D, et al. Detection of temozolomide-induced hypermutation and response to PD-1 checkpoint inhibitor in recurrent glioblastoma. Neurooncol Adv. 2022;4(1):vdac076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crisafulli G, Sartore-Bianchi A, Lazzari L, Pietrantonio F, Amatu A, Macagno M, et al. Temozolomide Treatment alters Mismatch Repair and boosts mutational Burden in Tumor and blood of Colorectal Cancer patients. Cancer Discov. 2022;12(7):1656–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ridolfi L, Petrini M, Granato AM, Gentilcore G, Simeone E, Ascierto PA, et al. Low-dose temozolomide before dendritic-cell vaccination reduces (specifically) CD4 + CD25 + + Foxp3 + regulatory T-cells in advanced melanoma patients. J Transl Med. 2013;11:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krug S, Abbassi R, Griesmann H, Sipos B, Wiese D, Rexin P, et al. Therapeutic targeting of tumor-associated macrophages in pancreatic neuroendocrine tumors. Int J Cancer. 2018;143(7):1806–16. [DOI] [PubMed] [Google Scholar]
- 30.Dost Gunay FS, Kirmizi BA, Ensari A, Icli F, Akbulut H. Tumor-associated macrophages and neuroendocrine differentiation decrease the efficacy of Bevacizumab Plus Chemotherapy in patients with Advanced Colorectal Cancer. Clin Colorectal Cancer. 2019;18(2):e244–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article.