Abstract
Organoids are “mini-organs” that self-organize and differentiate from stem cells under in vitro 3D culture conditions, mimicking the spatial structure and function of tissues in vivo. Extracellular vesicles (EVs) are nanoscale phospholipid bilayer vesicles secreted by living cells, rich in bioactive molecules, with excellent biocompatibility and low immunogenicity. Compared to EVs, organoid-derived EVs (OEVs) exhibit higher yield and enhanced biological functions. Organoids possess stem cell characteristics, and OEVs are capable of delivering active substances, making both highly promising for medical applications. In this review, we provide an overview of the fundamental biological principles of organoids and OEVs, and discuss their current applications in disease treatment. We then focus on the differences between OEVs and traditional EVs. Subsequently, we present methods for the engineering modification of OEVs. Finally, we critically summarize the advantages and challenges of organoids and OEVs. In conclusion, we believe that a deeper understanding of organoids and OEVs will provide innovative solutions to complex diseases.
Graphical Abstract
Keywords: Organoids, Extracellular vesicles, Organoids extracellular vesicles, Engineering modification, Disease treatment
Introduction
In the past few decades, classical cell lines have been the primary method for studying cells and have led to many medical breakthroughs. However, they have certain limitations that make them inadequate for many contemporary medical research needs. In particular, classical cell lines are challenging to use for certain exploratory and investigative experiments, such as pharmacological testing of various drugs for the treatment of complex diseases and the exploration of the origin and development of human organs. This is because classical cell lines lack the potential for differentiation, and their single lineage makes them unsuitable for research in modern biomedical studies [1]. Therefore, finding a new research model has become a significant challenge in the biomedical field. In recent years, the continuous development of stem cell technology has witnessed the increasing maturity of organoids technology, providing hope to the research community.
Organoids are 3D cell clusters cultivated from stem cells through in vitro 3D culture techniques. Organoids are capable of self-renewal and self-organization, and belong to a class of microphysiological systems, exhibiting similar spatial structures and physiological functions to source tissues. The process of cultivating organoids using stem cells from healthy/diseased tissues can recapitulate the characteristics of the source tissue, providing a possibility for studying the growth mechanisms of the source tissue/tumor [2, 3]. Organoids bridge the gap between 2D classical cell lines and traditional animal models by providing a more accurate and manipulative system than 2D cells. 2D cell cultures are direct contact between cells and the medium, while organoids realize frequent contact between cells, which has obvious advantages in simulating source tissues [4]. Organoid models address the limitation of 2D cell cultures in describing the entire physiological behavior of tissues, providing a novel approach for drug screening, testing, development, and disease research [5].
EVs, are nanosized vesicles secreted by living cells, serving as crucial mediators for intercellular information exchange [6–8]. Current research often focuses on extracting EVs from culture dishes. However, as 3D cell clusters, organoids exhibit a more complex level of communication among cells compared to 2D classical cell lines. Consequently, the complexity of OEVs extracted from organoids far surpasses that of EVs sourced from 2D cultures. Inheriting the minute nano size and excellent biocompatibility of EVs, and enhancing the complexity of their own active substances, OEVs hold vast prospects for medical applications.
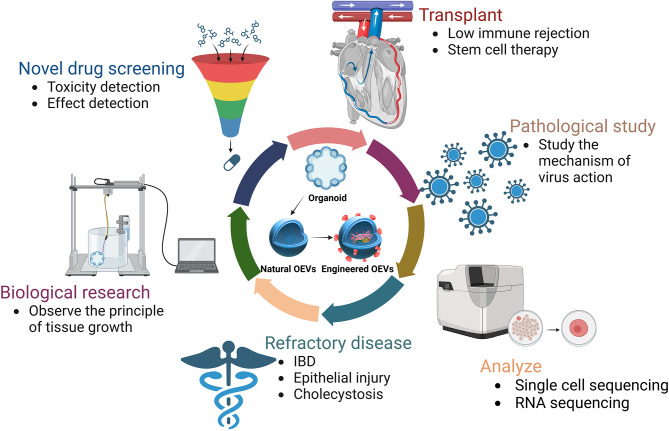
In this review, we will explore the roles of organoids and OEVs in disease treatment research and discuss the potential utility of organoids and OEVs in the future, aiming to enhance our understanding of the significance of these two innovative technologies. The applications of organoids and OEVs in the treatment of intricate disease is of vital significance, as illustrated in Fig. 1.
Fig. 1.
Schematic diagram of the applications of organoids and OEVs. Organoids and OEVs can be treated by oral administration, transplantation, injection and delivery of bioactive substances. The figure was created with https://app.biorender.com/
Overview of organoids
Organoids are 3D cell clusters that self-assemble and differentiate in an in vitro 3D environment, recapitulating the spatial structure and function of in vivo organs [9–11]. Organoids can be derived from embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), and adult stem cells (ASCs) [12, 13]. The self-assembly process of organoid formation is constrained by spatially restricted lineage commitment and cell sorting. Proper culture conditions and specific cytokines are critical mediators for organoid construction [14].
ASCs primarily originate from adult tissues, such as adult cells found in the skin or blood [15, 16]. ASCs have a relatively limited differentiation potential, therefore capable only of cultivating cell types associated with the source tissue, resulting in organoids with a singular physiological function [17, 18]. However, ASCs have the advantage of being widely sourced and easily obtainable.
In contrast, iPSCs and ESCs possess extensive differentiation potential, capable of differentiating into multiple diverse cell types, thereby mimicking various cell types found within the body [19, 20]. This capability enables organoids cultivated from iPSCs to exhibit more complex spatial structures and diverse physiological functions, enhancing their diversity [21, 22]. Therefore, organoids cultivated from iPSCs and ESCs are more suitable for applications in fundamental research and drug screening [23, 24].
Currently, organoids research is still in its infancy. The functions of the currently constructed organoids are relatively homogeneous, which was not the original purpose of organoids technology development. For example, if a fracture patient were to have a bone organoids transplant, the therapeutic effect of a single physiologically functioning bone organoids would be minimal. The ultimate goal of the development of organoids technology is to construct an organoids with multiple physiological functions (such as bone formation, bone resorption, hematopoiesis, and good mechanical properties) for better therapeutic outcomes [25].
Organoids for disease treatment
Organoids for bone defects
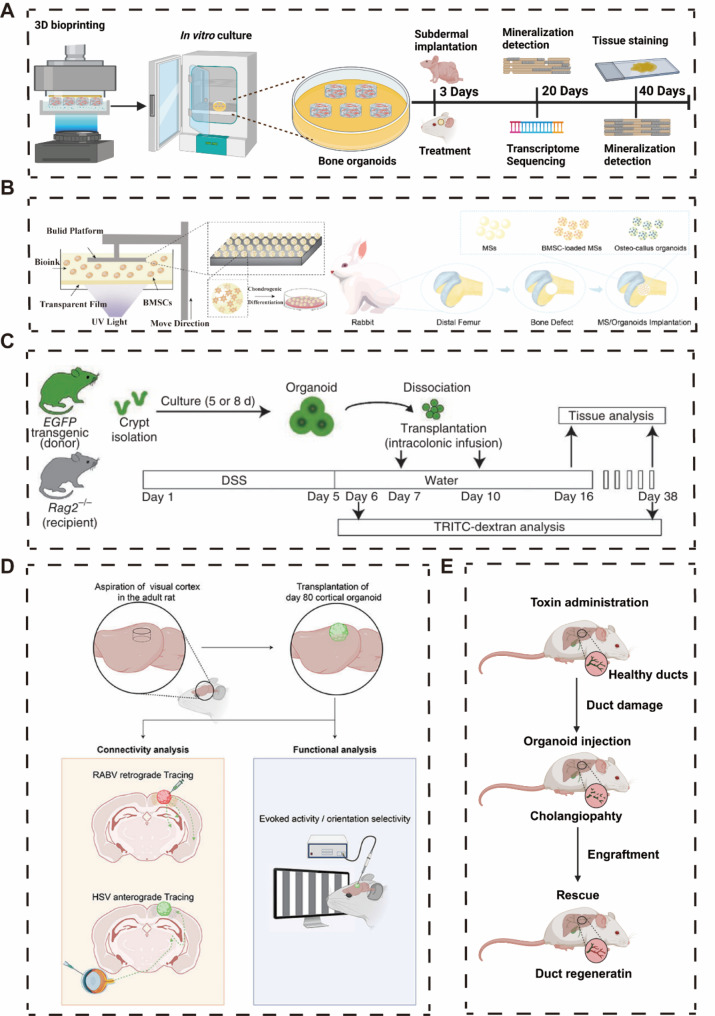
Wang et al. designed a novel bioink using Gelatin Methacryloyl (GelMA), Alginate Methacrylate (AlgMA), and Hydroxyapatite (HAP) to simulate the structure of bone tissue through bioprinting technology. According to Scanning Electron Microscopy (SEM) and cryo-SEM results, the bioprinted GelMA/AlgMA/HAP hydrogel exhibits a porous structure and uniform porosity suitable for osteoblast mineralization. During in vitro culture, it effectively promotes osteoblast mineralization, evidenced by significant mineral deposition. Upon implantation in vivo, GelMA/AlgMA/HAP undergoes self-mineralization and forms trabecular bone-like structures. This process accelerates osteoblast mineralization by stimulating signaling pathways related to mesenchymal stem cell osteogenic differentiation, including PI3K-Akt, Ras, Rap1, and MAPK. Consequently, there is a substantial increase in the expression of osteogenic markers such as ALP, OCN, OPN, and Run x2 (Fig. 2A) [26].
Fig. 2.
Applications of different organoids in disease treatment. A Bioprinted bone organoids for the treatment of cranial defects. B Bioprinted MS spheres, developed in vitro into callus organoids for the treatment of femoral defects. C Colonic organoids constructed using Lgr5 crypt stem cells, transplanted to the affected sites in IBD mice for treatment. D Transplantation of forebrain organoids for the treatment of neural injuries in mice. E Biliary organoids accelerating the healing of bile duct injuries in mice. (copyright [26, 34–37])
To address the issue of incomplete bone healing and long healing times, Professor Ouyang’s team at Zhejiang University developed Osteo-callus organoids to mimic callus formation for effective bone regeneration [27–30]. The primary mechanism for most bone tissue regeneration is endochondral ossification, in which mesenchymal stem cells are recruited at the site of injury and differentiate into callus [31]. Using digital light processing (DLP) technology, the team constructed osteo-callus organoids similar to those in bone development by agglutinating, culturing, and inducing differentiation of bone marrow mesenchymal stem cells (BMSCs) in microspheres [32, 33]. This organoid possesses a similar cellular composition to the endochondral ossification process and is capable of rapid in situ bone regeneration in just four weeks (Fig. 2B).
Organoids for IBD
As an autoimmune disease, inflammatory bowel disease (IBD) has no effective treatment, and only medication or surgical removal can be used to halt the progression of the disease [38, 39]. Shiro Yui et al. successfully constructed colon organoids using intestinal crypt Lgr 5 stem cells. By dissociating the colon organoids and transplanting them into the diseased regions of IBD mice via enema, they observed that the transplanted organoids successfully integrated into the affected areas and alleviated the severity of IBD to a certain extent. Examination of the expression of Ki67, Alcian blue, ChgA, CA2, and COX1 confirmed that the transplantation of colon organoids effectively improved cell proliferation, mucus secretion, and intestinal metabolism in the diseased regions of IBD mice (Fig. 2C) [35].
On July 7, 2022, Tokyo Medical and Dental University completed the world’s first clinical application of intestinal organoids to treat IBD. Throughout the procedure, no additional pharmacological treatments were used, relying solely on intestinal organoids derived from the patient’s own healthy stem cells. The patient showed positive recovery after the surgery, but the final treatment outcomes are pending further follow-up data [40]. Furthermore, based on the Japan Clinical Trial Registry (ID: jRCT b032190207), Tokyo Medical and Dental University is planning a clinical trial aimed at assessing the safety of transplanting autologous intestinal epithelial stem cells, cultured ex vivo, into patients with refractory ulcerative colitis. This trial will recruit 8 IBD patients over the age of 20. As part of the protocol, autologous intestinal epithelial organoids will be cultured for approximately five weeks, and following confirmation of their viability through specific testing, the organoids will be transplanted to the ulcerated regions of the patients using endoscopic techniques.
Organoids for nerve repair
After traumatic brain injury, long-term neurological impairments occur [41–43]. In the adult mammalian brain, neurogenesis and axon regeneration are regionally restricted and possess limited regenerative abilities [44, 45]. Previous research has identified cell transplantation as an effective method for restoring brain function. Transplanting fetal rodent cortex into the cortex of adult rodents can stimulate the growth of neuronal processes in adult rodents, and the transplant can integrate locally with the brain [46–49]. This demonstrates the potential of cell transplantation to reconstruct neuronal networks in the cerebral cortex.
Building upon this concept, combining brain organoids largely replicates the diversity of brain cell types and structure. Additionally, the development of forebrain organoids mirrors the timeline of normal neurodevelopment (generating radial glial cells, a fundamental laminar structure containing separated upper and lower cortical neuron layers, and detecting astrocytes and oligodendrocytes in the later stages of forebrain organoid development). Jgamadze et al. transplanted forebrain organoids into the injured visual cortex of adult rats. The post-transplant experimental results demonstrated remarkable systemic integration of the transplant with the host cortex and exhibited robust spontaneous neural activity (Fig. 2D) [36].
Organoids for cholangiopathy
Sampaziotis et al. isolated cells from three regions of the biliary tree—intrahepatic bile ducts (IHD), common bile duct (CBD), and gallbladder (GB)—and performing single-cell RNA sequencing, Uniform Manifold Approximation and Projection (UMAP) analysis revealed that cells from these sources share a common core transcriptional profile, while cholangiocytes from different regions exhibit unique gene expression characteristics. This indicates that cholangiocytes in different areas of the biliary tree possess distinct functions, adapting to varying microenvironments. Subsequently, cells from IHD, CBD, and GB were cultured under specific conditions to form organoids, and scRNAseq analysis was conducted on these cholangiocyte organoids [50, 51]. According to UMAP and Principal Component Analysis (PCA) analysis, transcriptional differences between cells vanished, showing overlapping transcriptional profiles, suggesting that cholangiocytes from different regions now share similar transcriptional characteristics. Moreover, when constructing organoids with cells from IHD, CBD, and GB, and intervening with gallbladder bile, scRNAseq analysis post-bile treatment revealed a new overlapping gene expression profile in the treated cholangiocyte organoids. This demonstrates that cholangiocyte organoids can acquire identities from different regions of the biliary tree when exposed to the appropriate microenvironmental changes, indicating a high plasticity of cholangiocyte organoids.
When cholangiocyte organoids were transplanted into the lesion sites of mice with cholangiopathy, mice that did not receive transplantation died within three weeks, while those receiving the transplanted cells survived up to three months, with the transplanted cholangiocytes occupying 25–55% of the regenerated biliary epithelium. Furthermore, MR imaging of the transplant areas showed no abnormal growth or tumor formation, suggesting that the transplantation of cholangiocyte organoids provided healthy cells for repairing the liver and biliary damage (Fig. 3E) [37].
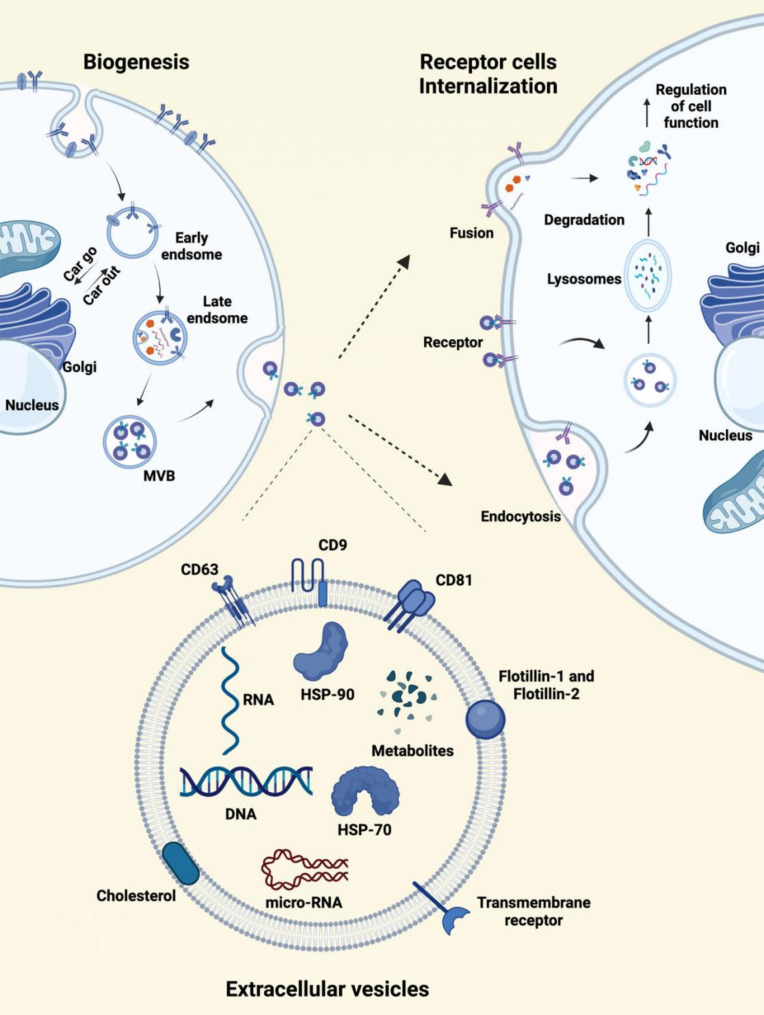
Fig. 3.
The biogenesis, structure, composition, and internalization of OEVs. The cytoplasmic membrane engulfs substances in the extracellular environment through endocytosis to form early endosomes. Early endosomes gradually mature into late endosomes through cargo exchange with the Golgi apparatus. Within the membranes of late endosomes, multivesicular bodies are formed, which contain luminal vesicles. With the help of related proteins, late endosomes either fuse with lysosomes for degradation or fuse with the plasma membrane to release the luminal vesicles that are called exosomes. OEVs can be taken up by recipient cells through membrane fusion, endocytosis, and receptor-mediated signaling pathways. OEVs are phospholipid bilayers with specific proteins and membrane receptors, and the membranes are rich in RNA, DNA, proteins, and some cellular metabolites. The figure was created with https://app.biorender.com/
In summary, Organoids, as emerging therapeutic tools, offer profound potential in both biomedical research and clinical applications, going beyond mere speculation. As complex 3D cell cultures that recapitulate key characteristics of organs, organoids provide a dynamic platform for modeling disease, testing therapeutics, and even potentially regenerating damaged tissues. Although still in relatively early stages of development, they are already demonstrating transformative capabilities in various fields of research. For instance, the Takebe team successfully created a fatty liver disease model by treating liver organoids with oleic acid, significantly advancing the study of non-alcoholic steatohepatitis and facilitating the development of targeted therapies [52]. This achievement underscores the utility of organoids in recreating complex disease environments, opening avenues for drug testing and personalized medicine approaches in metabolic and liver-related disorders. Similarly, Lancaster’s team developed a protocol for generating brain organoids that contain neural tissues and mature brain structures, marking a significant step forward in using organoids to study human tissue and organ development [53]. These advances underscore the vast potential of organoid technology in biomedical research and clinical applications. We also look forward to the continued development and maturation of organoid technology, hoping it will eventually be applied in clinical treatments to address complex diseases that modern medicine struggles to resolve.
The field of organoids research holds immense promise for expanding our understanding of human biology and advancing the treatment of complex diseases. As protocols become more standardized and reproducible, organoids could potentially bridge the gap between preclinical studies and human clinical trials. The ultimate goal is to refine organoid technology to a point where it can be utilized in clinical settings, offering solutions for organ transplantation, tissue regeneration, and the treatment of chronic conditions such as diabetes, cardiovascular diseases, and neurodegenerative disorders. As these systems evolve, they hold the potential to transform therapeutic approaches for a broad range of diseases that remain unresolved by conventional medicine, providing new hope for patients and driving forward the frontiers of personalized and regenerative medicine.
Overview of OEVs
OEVs are secreted by organoids. Like EVs secreted by traditional 2D cells, OEVs is responsible for information exchange and material transport between organoid cells, and is an important connecting medium for communication between organoid cells. OEVs contain abundant bioactive substances, such as proteins, RNA, DNA, etc., which can regulate gene expression and function of target cells [54–56].
Based on their origin, both OEVs and EVs are classified as mammalian extracellular vesicles (MEVs). As a result, they share many similarities in terms of biogenesis, internalization mechanisms, and composition. However, due to the unique three-dimensional structure of organoids and the complex diversity of cell types within them, there are significant differences between OEVs and traditional EVs. OEVs more closely resemble EVs isolated from human body fluids, as they better simulate the intricate cellular interactions and environmental conditions found in vivo.
Biogenesis, structures, composition, and internalization of OEVs
OEVs comprise three isoforms: microvesicles (100–1000 nm), exosomes (30–150 nm), and apoptotic bodies (50–5000 nm) [57, 58]. Among them, exosomes are a well-studied subtype because of their good biological function and application potential. In this review, OEVs also refer to exosomes unless otherwise specified.
OEVs are nanoscale vesicles secreted by cells into the extracellular compartment through the double invagination of the plasma membrane [59]. The biogenesis of OEVs occurs in three main stages (Fig. 4): (1) Cells form early endosomes by endocytosing surface and soluble proteins, which may merge with other early endosomes or internalize OEVs from other cells; (2) Early endosomes mature into multivesicular bodies (MVBs) as they accumulate intraluminal vesicles (ILVs) through interactions with the Golgi apparatus and endoplasmic reticulum, aided by the Endosomal·sorting complexrequired for transpor (ESCRT) complex; (3) MVBs either fuse with lysosomes for degradation or with the cytoplasmic membrane, releasing the ILVs as OEVs [60–63].
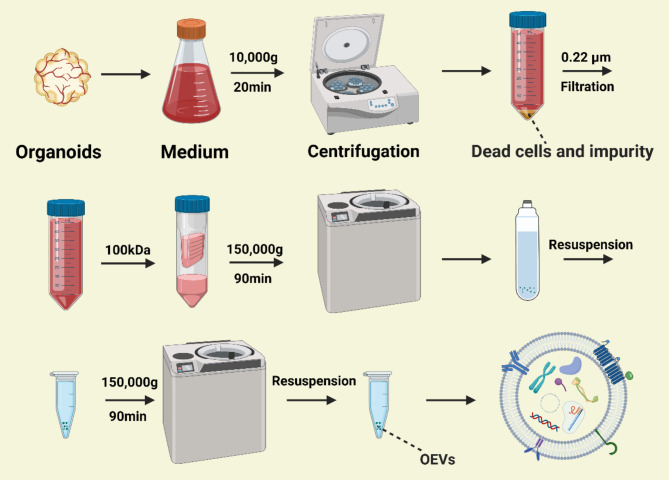
Fig. 4.
Workflow for the extraction of OEVs. Organoid-conditioned media were collected and centrifuged at 10,000 g for 20 min at 4 °C to remove dead cells and debris. The supernatant was filtered using a 0.22 μm filter and a 100 kDa ultrafiltration membrane. Subsequently, the filtrate was ultracentrifuged at 150,000 g for 90 min at 4 °C to obtain OEVs. The ultracentrifugation step was repeated to purify the OEVs. The figure was created with https://app.biorender.com/
OEVs transport bioactive substances to other cells, promoting communication through either membrane fusion or endocytosis (Fig. 3) [64, 65]. When fusion occurs, phospholipid bilayers of OEVs and target cells create a semi-fused stalk, which expands into a hemifusion diaphragm, eventually allowing complete fusion [66, 67]. Endocytosis, particularly clathrin-mediated and caveolin-dependent pathways, is another major mechanism for OEV internalization [68–71].
Isolation of OEVs
Compared to traditional cell lines, organoids require more complex culture conditions, typically involving three-dimensional scaffolds such as Matrigel. This complexity introduces additional challenges in the isolation of OEVs, as proteins or other components within the culture matrix can interfere with the separation process. Moreover, the use of enzymes to digest the matrix during the removal process may negatively impact the integrity of the OEVs. As a result, the isolation of OEVs requires more precise and delicate handling than that of traditional EVs to ensure the purity and functionality of the vesicles are preserved without contamination or degradation.The commonly used separation methods are Differential centrifugation, Density gradient centrifugation, Ultracentrifugation, Ultrafiltration, Immunoaffinity capture and Size exclusion chromatography [72, 73]. As shown in Table 1, different OEVs isolation methods have their own advantages and disadvantage.
Table 1.
Common methods of OEVs isolation and their advantages and disadvantages
| Methods | Advantages | Disadvantages | Reference |
|---|---|---|---|
| Differential centrifugation | Simple operation, economy | Low purity, long time | [81] |
| Density gradient centrifugation | High purity, high yield |
High cost, long time, high requirements for instruments |
[82] |
| Ultracentrifugation | High yield, Simple operation | Low purity, long time | [83] |
| Ultrafiltration | Simple operation, efficient, high yield | Membrane blockage | [84] |
| Immunoaffinity capture | High purity, high specificity | High cost, lack of markers, | [85] |
| Size exclusion chromatography | High purity, Simple operation | Long time, high cost | [86] |
Our team has developed a method for isolating BEVs that is applicable to most bacteria, including both Gram-negative and Gram-positive species [74]. This method has also proven effective for the isolation of MEVs [75, 76]. Building on this approach, we have established a new protocol for the extraction of OEVs. Here, we emphasize that in order to achieve optimal OEV isolation, organoid culture medium should be free of EVs, specifically using EV-depleted serum to prevent contamination by external EVs [77–79].
The OEV extraction protocol is as follows: The organoid culture medium is replaced with fresh conditioned medium 24–48 h before collection to avoid interference from components in the culture matrix. The organoid-conditioned medium is then collected, and OEVs are extracted and isolated within 24 h. First, the medium is subjected to low-speed centrifugation to remove residual cells and impurities. The supernatant is then filtered using a 0.22 μm sterile filter, followed by ultrafiltration using a 100 kDa filter to eliminate extraneous proteins. Subsequently, two rounds of ultracentrifugation are performed to isolate and extract the OEVs, as shown in Fig. 4.
Characterization of the isolated OEVs differs from that of traditional EVs. Although Transmission·Electron·Microscope (TEM) and Nanoparticle Tracking Analysis (NTA) are routinely employed for evaluating OEV morphology, size, and concentration, the presence of multiple cell types in organoids leads to a more complex and diverse range of OEVs in terms of both types and functions. Thus, deeper consideration is required when performing specific analyses such as Western blotting (WB) or fluorescent capture of surface marker proteins, depending on the cell type origin of the OEVs [80]. This diversity necessitates further optimization and refinement of detection methods to ensure the accuracy and scientific validity of the findings, marking a key distinction from traditional EVs isolation.
The difference between OEVs and EVs
The primary difference between organoids and conventional cell cultures is that the former have a 3D structure, while the latter are 2D. Cells in a 3D environment exhibit much more complex communication than those in a 2D environment, and this increased complexity promotes the complexity of EVs.
Existing studies support this conclusion. For instance, when human Mesenchymalstem cells (hMSCs) are cultured in a 3D-focused state, the secreted 3D-hMSC-EVs have a higher yield and increased CD63 expression [87]. Additionally, another report found that EVs secreted from 3D MSCs have an average concentration 28 times higher, with significantly increased expression of CD81 and CD9. These 3D-MSC-EVs can induce greater neurite outgrowth and branching, thereby better promoting nerve regeneration. This suggests that 3D-MSC-EVs possess more potent biological functions [88]. A study demonstrated that exosomes secreted by mesenchymal stem cells (MSCs) cultured in a 3D environment could be used in the treatment of Alzheimer’s disease. The researchers also observed significant differences in miRNA and protein levels between exosomes derived from 3D cultures (3D-EVs) and those from traditional two-dimensional cultures (2D-EVs) [89]. Anotherr study found that cells isolated from two gastric cancer (GC) cell lines were cultured in 2D and 3D conditions, and EVs released from cells in 2D and 3D conditions were collected. It was observed that GC cells in 3D secreted a higher number of smaller EVs and had a downregulation of ADP-ribosylation factor 6 and an overall upregulation of microRNA compared to cells in 2D conditions [90]. In studies utilizing 3D-MSC-EVs for cardiac repair, it was found that 3D-MSC-EVs applied to the border zone of myocardial infarction exhibited significantly stronger cardioprotective effects compared to 2D-MSC-EVs. This finding further indicates that 3D culture significantly enhances the activity of EVs [91]. Another study indicated that the secretome of bone marrow-derived MSCs facilitated corneal wound healing, implying that the 3D culture system could enhance the secretome and optimize the cell culture environment. Consequently, MSCs cultured on 3D fibers may create a favorable microenvironment, leading to the production of high-quality EVs with potent therapeutic effects on acute myocardial infarction (AMI) [92]. In addition, mice were injected with MSC derivatives isolated from 2D and 3D cultures, respectively, to observe the recovery of TBI disease in mice after injection. It was found that mice injected with MSCs derived from 3D organoids cultures had better recovery in all aspects of angiogenesis and neurological recovery than mice injected with MSCs in 2D [88, 93]. This change in the number and cargo properties of EVs is likely since cells in 3D culture conditions have a more natural physiological structure and secreted EVs are more like living bodies than those secreted in 2D [94].
However, it is important to acknowledge that the aforementioned findings are based on EVs derived from 3D cell cultures, which still differ from OEVs. 3D cell cultures involve a single cell type and have a simple structure. In contrast, the OEVs we describe are not simply EVs secreted from cells in a 3D environment; they are secreted by organoids that self-assemble and differentiate into complex spatial structures with multiple cell subtypes. We have reason to believe that OEVs will have higher yields and better biological functions than 3D-EVs. This idea is not unfounded. Compared to 2D cell cultures, 3D cell cultures increase the contact area between cells and enhance intercellular communication, which gives 3D-EVs their various advantages over 2D-EVs. Organoids, compared to regular 3D cell cultures, not only expand the intercellular contact area but also enrich the diversity of cell subtypes, greatly increasing the complexity of intercellular communication. Consequently, the complexity of OEVs is expected to surpass that of 3D-EVs.
Applications of OEVs
EVs is a cell-free system that delivers active substances to participate in intercellular communication, which is an excellent therapeutic agent in itself and a good delivery carrier [95–97]. OEVs can be used for the treatment of various diseases and for the delivery of target genes. Additionally, OEVs accurately reflect the growth conditions of organoids. When combined with organoid technology, OEVs facilitate drug screening, disease research, and cancer research. Furthermore, OEVs can overcome natural physiological barriers in the human body, making them excellent drug delivery carriers.
Intestinal OEVs for immunomodulatory
The results of many studies show that EVs of multiple origins have immunomodulatory abilities [98, 99]. For example, EVs from the intestinal epithelium can regulate Treg cells [100, 101]. Meanwhile, the secreted OEVs from both mouse intestinal stem cells and/or intestinal organoids grown from human intestinal crypt stem cells have significant immunomodulatory ability and can significantly reduce the production of various inflammatory factors induced by LPS. Moreover, when morphine was used to intervene in the organoids, OEVs ceased to have immunomodulatory capacity (Fig. 5A). After analyzing and comparing OEVs before and after morphine treatment, it was found that multiple microRNAs, especially Let 7, are key components of the immunomodulatory ability of OEVs [102, 103]. In particular, Let 7 is a key molecular regulator of inflammation control [104].
Fig. 5.
Applications of different OEVs in disease treatment. A OEVs secreted by intestinal organoids exhibit anti-inflammatory effects. B OEVs secreted by salivary gland organoids promote epithelial wound healing. C Müller glial cells derived from retinal organoids treat retinal diseases by secreting OEVs. D OEVs derived from retinal organoids regulate lipid metabolism to treat retinal diseases. (copyright [77, 79, 104, 108])
Salivary glands OEVs for epithelial repair
Ferreira et al. designed a magnetic 3D Bioassembly (M3DB) platform that enables the safe and non-invasive 3D culture of hDPSCs labeled with magnetic nanoparticles and the construction of functional Salivary glands organoids (SGo) [79]. The constructed SGo exhibited extensive expression of SG-specific markers (AQP1, MUC7, KRT5, KRT14, CHRM3, TUBB3), demonstrating the success of the M3DB platform in SGo construction. Next, EVs secreted by 3D-hDPSC and SGo were extracted, resulting in 3D-hDPSC-EVs and SGo-OEVs, which were tested for their reparative abilities in an epithelial injury model. The evaluation criteria included epithelial cell growth and neuronal repair effects. The therapeutic efficacy of 3D-hDPSC-EVs was 15%, direct SGo transplantation was 25%, whereas the use of SGo-OEVs achieved up to 60% therapeutic efficacy. Proteomic analysis revealed the abnormal expression of 99 specific proteins in SGo-OEVs, many of which are closely related to biological regulation, adhesion, biogenesis, development, and cell growth. This is an exciting result, clearly demonstrating the differences between 3D-EVs and OEVs, with the biological functions of OEVs being far superior to those of 3D-EVs. Interestingly, the therapeutic effect of OEVs surpassed that of direct SGo transplantation (Fig. 5B). Currently, it is unclear whether this is a special phenomenon or a general occurrence. We will closely monitor further research on the roles of OEVs and organoids in disease treatment.
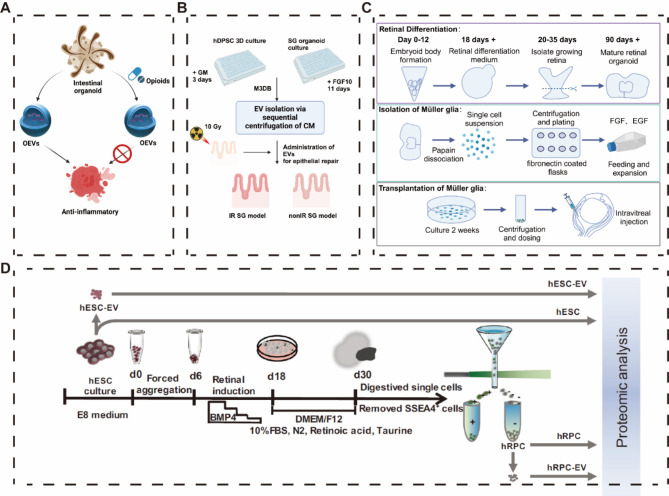
Retinal organoids OEVs for retinal degeneration repair
Currently, experiments are demonstrating the therapeutic benefits of OEVs. In one experiment, retinal organoids were generated in vitro using hESC for the treatment of retinal degenerative diseases, and Müller glial cells (a type of retinal cell with stem cell properties related to retinal homeostasis) expressing CD29 (β1 integrin receptor and fibronectin ligand) were used to selectively enrich Müller glial cells by adhering cells isolated from retinal organoids to fibronectin-coated culture plates [105–108]. Intravitreal injection of Müller glial cells isolated from retinal-like organs into Retinal Ganglion Cell mice showed a partially significant recovery of visual function 4 weeks after transplantation [109]. Müller glial cells may be neuroprotective on the one hand by releasing neurotrophic factors. However, these neuroprotective factors may be transient, and to a greater extent, OEVs released by Müller glial cells are likely potentially neuroprotective by controlling neuronal apoptosis and survival (Fig. 5C).
The Xu research team constructed retinal organoids (hERO) using ESCs and treated retinal degeneration (RD) by transplanting hERO-derived retinal progenitor cells (RPC) [110]. Stem cell transplantation has been one of the best treatment options for many diseases, but it still faces challenges such as low survival rates and immune rejection. EVs-mediated cell-free therapy is gradually showing its unique charm [111, 112]. To this end, they extracted hESC-EVs and hERO-RPC-EVs. The results showed significant differences in the proteomic profiles of the two types of EVs. Most secreted and luminal proteins were specifically enriched in hERO-RPC-EVs. Furthermore, the enrichment levels of EVs markers also differed between the two types of EVs. For example, CD9, ACTB, GAPDH, HSPA8, and TUBA1C were highly expressed in hESC-EVs but low in hERO-RPC-EVs, while TSG101, FN1, and collagen family members exhibited the opposite pattern. hERO-RPC-EVs were rich in fatty acid-specific transport proteins and enzymes related to fatty acid metabolism, showing stronger lipid precipitation processing capabilities than hESC-EVs. In contrast, hESC-EVs were enriched with many proteins related to cell division and proliferation. hESC-EVs could promote angiogenesis but carried the risk of abnormal proliferation and tumor formation, whereas hERO-RPC-EVs did not contain similar risk proteins (Fig. 7D). Their unique proteins could improve lipid metabolism, potentially offering a novel approach to treating RD by regulating lipid metabolism [77].
Singh Mandip’s study showed that OEVs secreted by retinal-like organs have superior quantitative advantages and rich nanomechanical properties (OEVs are softer and more rounded than EVs) compared to EVs secreted by human umbilical cord mesenchymal stem cells (hUCMSC). Late stage organoids (Day > 120, fully differentiated) secrete more OEVs than early stage organoids (Day 50-Day 90, rich in retinal stem cells) [113]. In addition, the remaining studies found that OEVs secreted by retinal-like organs were associated with the regulation of retinal [114, 115]. OEVs have the potential to transport miRNAs from their membranes to retinal progenitor cells (hRPCs) [116]. Thus, retinal organoids OEVs may be a key mediator in the treatment of retinal degenerative diseases [117].
Epidermal organoids OEVs for epithelial injury repair
The Lee team successfully utilized iPSCs to create epidermal organoids (iEpiO) and cultured them as a monolayer of epidermal cells on 2D cell culture plates, losing their organoid characteristics (Fig. 5D). Subsequently, EVs secreted by iEpiO under both 3D and 2D culture conditions were extracted. NTA results showed that the number of EVs obtained under 3D conditions was twice that of 2D, with a similar average particle size. CD9 expression increased, while Alix and Tsg101 expression decreased. Additionally, EVs from the 3D culture contained a large amount of vascular endothelial growth factor (VEGF). PCR and tube formation assays confirmed that EVs from the 3D culture significantly stimulated VEGF mRNA expression in Human·Wmbilical Vein Endothelial Cells (HUVECs) and promoted angiogenesis. sRNA-seq results indicated that 16 miRNAs were upregulated in 3D-EVs. These upregulated miRNAs primarily targeted genes involved in cell proliferation, migration, development, epithelial differentiation, and angiogenesis. This was further confirmed by a mouse skin wound healing experiment. On days 3, 5, and 7, mice treated with 3D-EVs injections showed an average enhancement of 1.6 times in skin healing rate. Immunohistochemistry (IHC) staining also demonstrated that 3D-EVs promoted high expression of VEGF at the treatment site [118].This is a fascinating phenomenon: when organoids are transferred from a 3D to a 2D state, many organoid characteristics disappear, most notably the spatial structure of the organoid. There are significant differences in the expression of many markers between the two, and the biological functions exhibited by the obtained EVs also show notable differences.
In conclusion, OEVs hold significant potential for medical applications, effectively addressing the limitations of traditional EVs, such as low extraction efficiency and insufficient functionality. In fact, OEVs exhibit even stronger physiological effects in certain aspects compared to the organoids themselves. As the field of organoid technology continues to evolve, it is expected to drive further advancements in OEV research. The development of more sophisticated organoid models, such as vascularized and innervated organoids, is likely to result in OEVs with even more specialized functionalities, tailored for specific medical applications. For example, vascularized organoid-derived EVs could be used to enhance angiogenesis in ischemic tissues, while innervated organoid-derived EVs might hold promise for treating neurodegenerative conditions by promoting neuronal repair and regeneration.
The future of OEV-based research is likely to benefit from the integration of other cutting-edge technologies, such as CRISPR/Cas9-mediated gene editing and 3D bioprinting. By genetically engineering organoids to produce OEVs with specific therapeutic properties, researchers can create highly targeted treatment modalities for a variety of diseases. Additionally, 3D bioprinting may allow for the creation of more physiologically relevant organoids, further enhancing the functionality and therapeutic potential of the OEVs they produce. OEVs hold significant potential for advancing biomedical science, offering a versatile and powerful tool for disease modeling, therapeutic development, and precision medicine. Their ability to overcome the limitations of traditional EVs, combined with their unique functional properties, positions OEVs as a promising platform for future medical applications. As organoid technology continues to advance, it will undoubtedly drive further breakthroughs in OEV research, paving the way for new treatments and interventions for some of the most challenging diseases faced by modern medicine.
As organoid technology continues to evolve and improve, it will undoubtedly drive further advancements in OEV-related research. These small vesicles may have a profound impact on the future of biomedical science.
Engineering approaches for modifying OEVs
OEVs hold great potential as novel therapeutic agents and innovative nano drug delivery systems, but they still have limitations, such as a lack of targeting ability [119, 120]. Numerous articles have reported that engineering modifications can endow EVs with new functions [121, 122]. Due to the similarity between OEVs and EVs, engineering strategies are also applicable to OEVs, thereby granting them new functionalities to achieve specific goals, mentioned in Fig. 6. Here, we summarize the engineering modification approaches for OEVs, including modifications to organoids to indirectly obtain engineered OEVs and direct engineering of isolated OEVs. In Table 2, we summarize the methods and specific descriptions of engineering OEVs.
Fig. 6.
Methods for engineering OEVs and their applications. OEVs can be engineered through parental engineering, chemical modification, membrane fusion, fusion protein strategies, and electroporation. Both natural and engineered OEVs can be administered via oral intake, intravenous injection, local injection, and transdermal delivery. The figure was created with https://app.biorender.com/
Table 2.
Engineering transformation method of OEVs and its specific description
| Method | Specific description | Reference |
|---|---|---|
| Engineering parental | Organoids are engineered using genetic modification so that the OEVs they release acquire new functions | [124] |
| Chemical Engineering | Conjugating antibodies, peptides, or small molecule drugs to the OEVs surface to enhance targeting to specific cells or tissues | [125, 126] |
| Membrane Fusion | Using membrane fusion techniques to integrate the contents of liposomes or other nano-carriers containing drugs or genes into OEVs | [127] |
| Fusion Protein Program | By fusing proteins, OEVs can be fused with other active substances to give OEVs new functions | [128] |
| Electroporation Technology | Using electroporation to create temporary pores in the OEVs membrane, allowing large molecules or nucleic acids to enter the OEVs | [129, 130] |
Engineering parental organoids
Parental modification has been one of the effective methods for obtaining engineered EVs. For example, Liu et al. constructed ECN-pClyA-BMP-2-CXCR4 using synthetic biology techniques, fusing BMP-2 and CXCR4 to the ECN membrane via ClyA. Consequently, the produced BEVs’ membranes similarly featured BMP-2 and CXCR4 linked through ClyA. Lin et al. engineered HUVECs to overexpress Programmed death ligand 1 (PD-L1), and the EVs extracted from these cultured, genetically modified HUVECs inherited the overexpression of PD-L1 [130]. Modifying organoids is also likely to be an effective means of obtaining engineered OEVs.
In the context of using iPSCs to construct organoids, intercellular communication plays a crucial role in determining differentiation and morphogenesis. Tools designed for custom cell-to-cell communication are powerful assets in engineering organoids [131]. Currently, there are custom synthetic receptors engineered using synthetic biology, linking extracellular recognition to intracellular signaling, enabling targeted communication between cells. For instance, the synthetic Notch-based system (synNotch) facilitates cellular interactions [10, 132]. In this system, recipient cells expressing synNotch specifically recognize user-defined antigens. Upon synNotch receptors identifying the target antigen, engineered transcription factor regulation is triggered, enabling the engineered customization of organoids.
The field of artificially engineering and redesigning organoids is in its early stages. Presently, the creation of synthetic biological macromolecules such as proteins or nucleic acids is a relatively mature field. Supported by this mature technology, synthetic biology can design more complex biological tissues and achieve more intricate physiological functions [133]. However, concerning the design of engineered organoids, this is merely one cog in the entire system. Only when each cog is meticulously designed and the interplay between these components is achieved can we create engineered organoids with higher complexity and stronger physiological capabilities .
We can obtain OEVs that overexpress a certain protein after genetic modification of the organoids. By means of synthetic biology and other means, engineering organoids are constructed to influence the structural complexity and functional complexity of organoids, and OEVs with higher complexity functions are obtained.
Engineering BEVs after isolation
For isolated OEVs, the main engineering transformation targets revolve around membrane transformation and content transformation.
Chemical engineering
The membrane surface of OEVs was modified by covalent and non-covalent reactions [134, 135]. For example, DSPE-PEG-Mal-Cys-SDSSD (a class of bone-targeting peptides) was inserted into the phospholipid bilayer of OEVs by direct incubation with OEVs through hydrophobic forces, allowing OEVs to acquire bone-targeting ability [136]. On the other hand, covalent means such as click chemistry, aldehyde amine condensation and bioconjugation are effective solutions for membrane modification of OEVs [137].
Taking advantage of the characteristics of the amine fusion on the OEVs membrane being modified by the alkyne group, click chemistry can be well used for membrane modification of exosomes. When the alkyne group labels the amine group on the OEVs membrane, the azide-alkyl cyclization reaction enables the orthogonalization of OEVs proteins with azide compounds, thus imparting new properties to the OEVs membrane [124].
Membrane fusion
Benefiting from the characteristics of the phospholipid bilayer of OEVs, OEVs can spontaneously fuse with other membrane structures, thereby giving OEVs new functions [138, 139]. For example, OEVs are co-incubated with liposomes to fuse for new functions. Traeste et al. used strong cationic charge to induce OEVs to fuse with liposomes, thereby enhancing the binding ability of OEVs to recipient cells [140].
Fusion protein program
To load certain exogenous substances into EVs, the use of fusion proteins is a simple method. For example, Liu’s team used CD9-HuR fusion protein to co-incubate miR-26a-5p with EC-Exos (endothelial cell-derived EVs), achieving the introduction of miR-26a-5p into EC-Exos and enhancing its ability to contribute to bone differentiation [127, 141, 142].
Electroporation technology
By applying a high-intensity electric field force to achieve instantaneous increase in the permeability of the phospholipid bilayer and absorption of surrounding exogenous substances [129]. It can realize the transfer of DNA, RNA, and protein into OEVs [143]. Most critically, the electroporation technique does not change the physical properties of the siRNA transferred into OEVs [128].
Advantages and challenges of organoids and OEVs
Advantages and challenges of organoids
Understanding the development of basic tissues, biological organs, and many diseases has long been a challenge in biomedical research, with traditional cell lines and animal models offering limited assistance. However, advancements in organoid technology have opened new avenues for these complex studies. Organoids are 3D cell models that mimic the structure and function of the source tissue. Organoids derived from ASCs are constructed by directly dissociating the tissue of interest and culturing it for extended periods in the presence of tissue-specific growth factors. This allows them to simulate various diseases and has already been applied to cancer modeling. On the other hand, organoids derived from PSCs closely replicate the growth and developmental processes of tissues and organs, offering immense promise for studying human development without the limitations of tissue accessibility [144, 145].
Organoid technology also offers new opportunities for clinical development, serving as a bridge between animal models and human trials. By generating organoids from patient cells, personalized drug screening platforms can be created to identify the most effective treatment options for individual patients [146]. Moreover, organoids can be cultured at high density in microplates, facilitating high-throughput drug screening and rapidly generating data on various drug combinations [147–149]. The long-term viability of organoids in vitro provides an ideal model for studying chronic drug responses, helping researchers understand drug toxicity and resistance mechanisms. Organoid transplantation is the ultimate goal of this technology. Organoids derived from a patient’s own cells can significantly reduce immune rejection, and due to their stem cell-derived nature, they possess strong proliferative and differentiation potential, offering the prospect of tissue regeneration. As organoid technology continues to evolve, direct organoid transplantation may enable the regeneration of failing organ functions, the defeat of cancer, and rapid wound healing, representing a transformative new medium for clinical treatment.
However, despite the promising clinical applications and scientific research opportunities offered by organoid technology, it faces numerous problems and challenges. One key challenge is the lack of standardized criteria for organoids cultivation, which has resulted in relatively limited functional diversity in current organoids models. For example, bone organoids developed to date are only able to perform a single bone function, such as bone formation, bone absorption, or hematopoiesis [25, 150, 151]. Constructing multifunctional bone organoids that integrate these diverse functions poses a major challenge due to the complexities in directing stem cell differentiation [28]. Furthermore, the current bone organoids lack a rich vascular network necessary for proper physiological function, rendering them susceptible to necrosis owing to inadequate oxygen supply and waste accumulation [152]. Another limitation of current organoids technology is the lack of immune and nervous system integration, which is necessary for constructing more realistic and functional models of source tissues [153, 154]. Overcoming these challenges will require continued efforts to improve the methods for culturing, differentiating, and integrating different cell types into organoids, as well as the development of new technologies for controlling their growth and function (Fig. 7).
Fig. 7.
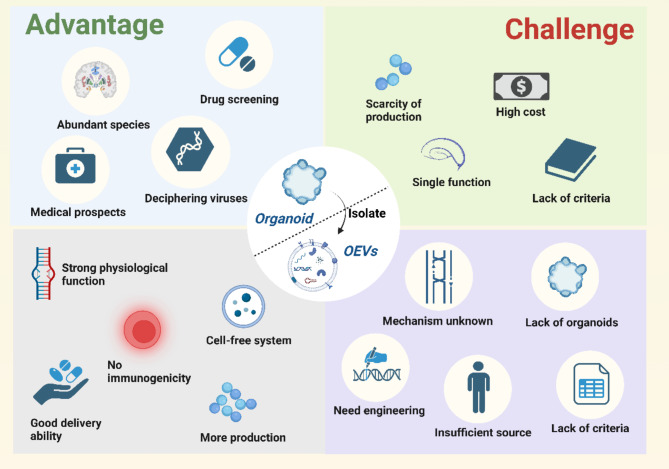
Advantages and challenges of organoids and OEVs. Organoids have unique advantages for tissue structure reduction, drug screening, medical applications, and virus decipherment, but also face a number of challenges such as low yields, single functions, high culture costs, and lack of standardized preparation guidelines; OEVs has its own advantages in relative quantity, physiological efficacy, immunogenicity, cell-free system, and drug delivery ability. At the same time, the major challenges of OEVs are the unclear mechanism of physiological effects, the insufficient number of organoids, the need for engineering transformation, the lack of abundant sources and the lack of standardized criteria. The figure was created with https://app.biorender.com/
Advantages and challenges of OEVs
OEVs are nanoscale phospholipid vesicles secreted by organoids, presenting significant potential in biomedical research. OEVs inherit many advantages of traditional EVs, such as being rich in bioactive molecules that regulate gene expression in target cells, while also offering low immunogenicity, a cell-free system, and efficient delivery capabilities. Furthermore, OEVs exhibit higher yields and enhanced physiological efficacy compared to conventional EVs. Their suitability for engineering modifications further expands their application potential.
However, several challenges persist. Although OEVs show improved yield relative to traditional EVs, their absolute production remains a considerable obstacle. The yield of OEVs is largely dependent on the number of organoids, and their quality is tightly linked to that of the organoids themselves. The limitations encountered in advancing organoid technology also restrict the development of OEVs. Moreover, natural OEVs exhibit certain limitations, including suboptimal efficacy and lack of targeting specificity, necessitating further engineering to enhance their therapeutic potential. The precise mechanisms by which OEVs exert their biological effects also remain poorly understood. While proteins and RNA are critical to OEV function, the exact molecular pathways involved are yet to be fully elucidated. Importantly, both organoid and OEV technologies are still in their infancy, with the lack of standardized protocols posing a significant barrier to the broader application of OEVs in research and clinical settings (Fig. 7).
Conclusions and perspective
With the development of technologies, the need to find new treatment options to address serious diseases is also increasing. So far, the transplantation of intestinal organoids, salivary gland organoids and lacrimal gland organoids has achieved satisfactory results in clinical application. In addition, organoids, as excellent 3D models, have unique advantages in drug screening, and many drugs have been successfully marketed after drug screening through organoids. Meanwhile, the Tokyo Medical and Dental University in Japan has utilized human intestinal crypt stem cells to construct intestinal quasi-organs, which have been employed in clinical treatments for IBD. This has lasssid the foundation for the clinical translation of organoids and OEVs. Our research team has also proposed the prospective applications of organoids and OEVs in the treatment of various diseases, anticipating the transformative impact this novel therapeutic approach will bring to clinical treatment [155].
This paper reviews the main features and construction of organoids, as well as the potential of organoids in the treatment of diseases, especially in liver and gallbladder diseases, bone injury and IBD. In addition, we are pleased to introduce OEVs [155], a novel EV that differs from bacterial-derived EVs [156–158], plant-derived EVs [159–161], and mammal-derived EVs. In this review, the biogenetic mechanism, composition, structure, internalization and separation methods of OEVs were reviewed, and the differences between OEVs and traditional EVs were highlighted. Further, we propose several engineering transformation methods based on OEVs structural characteristics to enhance the application effect of OEVs. The primary concept is to modify the membrane surface or contents to enhance the targeting of OEVs and/or to confer additional physiological functions. Most importantly, we highlight the high yield and strong physiological effects of OEVs, and describe the excellent effects of OEVs in the regulation of inflammation, the treatment of epithelial damage, and the treatment of degenerative retinal diseases [162].
In conclusion, we emphasize that both organoids and OEVs represent not only advanced models for biomedical research but also hold immense therapeutic potential, offering novel strategies and insights for the treatment of various diseases. However, before these technologies can be commercially viable, further optimization of their production processes is required. Future research should focus on improving organoid culture techniques, enhancing functional diversity, and employing innovative approaches to construct functionally integrated organoids capable of meeting the demands of clinical transplantation and treatment. At the same time, the methods for constructing organoids should be diversified, with a deeper exploration into their growth and developmental mechanisms. This will not only advance our understanding of human tissue and organ development but also provide ideal models for drug screening and personalized medicine. By expanding the applications of organoid technology, we can accelerate drug discovery and improve therapeutic outcomes. The ongoing advancements in organoid technology will directly contribute to the progress of OEVs, improving their yield and quality, and enhancing their physiological functionality, thereby further expanding their potential applications in biomedical fields. We hope this review highlights the immense potential of these cutting-edge technologies and inspires further research and exploration in this evolving field.
Acknowledgements
This work was supported in part by grants from the National Natural Science Foundation of China (82230071, 82202344); Integrated Project of Major Research Plan of National Natural Science Foundation of China (92249303); Shanghai Committee of Science and Technology Laboratory Animal Research Project (23141900600); Jiangsu Province Natural Science Foundation project (BK20241808).
Abbreviations
- EVs
Extracellular vesicles
- OEVs
Organoids extracellular vesicles
- ESCs
Embryonic stem cells
- iPSCs
Induced pluripotent stem cells
- ASCs
Adult stem cells
- GelMA
Gelatin Methacryloyl
- AlgMA
Alginate Methacrylate
- HAP
Hydroxyapatite
- SEM
Scanning Electron Microscopy
- DLP
Digital light processing
- BMSCs
Bone marrow mesenchymal stem cells
- IBD
Inflammatory bowel disease
- IHD
Intrahepatic bile ducts
- CBD
Common bile duct
- GB
Gallbladder
- UMAP
Uniform Manifold Approximation and Projection
- PCA
Principal Component Analysis
- MEVs
Mammalian EVs
- MVB
Multivesicular bodies
- ILVs
Intraluminal vesicles
- MEVs
Mammalian extracellular vesicles
- ESCRT
Endosomal sorting complex required for transpor
- TEM
Transmission Electron Microscope
- NTA
Nanoparticle Tracking Analysis
- WB
Western boltting
- hMSCs
Human Mesenchymal stem cells
- MSCs
Mesenchymal stem cells
- GC
Gastric cancer
- AMI
Acute myocardial infarction
- M3DB
Magnetic 3D Bioassembly
- SGo
Salivary glands organoids
- hERO
Human retinal organoids
- RD
Retinal degeneration
- RPC
Retinal progenitor cells
- hUCMSC
Human umbilical cord mesenchymal stem cells
- IEpiO
Epidermal organoids
- VEGF
Vascular endothelial growth factor
- HUVECs
Human Umbilical Vein Endothelial Cells
- IHC
Immunohistochemistry
- SnyNotch
Synthetic Notch-based system
- PD-L1
Programmed death ligand 1
Author contributions
Z. worte the original manuscript draft. L. S. and H. reviewed the manuscript. Z. W. and L. revised the manuscript. S. contributed to the conception and approved the submitted manuscript. All authors read and approved the submitted version.
Funding
This study was supported in part by grants from the National Natural Science Foundation of China (82230071, 82202344); Integrated Project of Major Research Plan of National Natural Science Foundation of China (92249303); Shanghai Committee of Science and Technology Laboratory Animal Research Project (23141900600).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have approved the manuscript be submitted.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guangyin Zhou, Ruiyang Li, Shihao Sheng and Jingtao Huang contributed equally to this work.
Contributor Information
Fengjin Zhou, Email: xarcsh@164.com.
Yan Wei, Email: ywei@shu.edu.cn.
Han Liu, Email: liuhanqiu@shu.edu.cn.
Jiacan Su, Email: drsujiacan@163.com.
References
- 1.Schutgens F, Clevers H. Human organoids: tools for understanding Biology and Treating diseases. Annu Rev Pathol. 2020;15:211–34. [DOI] [PubMed] [Google Scholar]
- 2.Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345:1247125. [DOI] [PubMed] [Google Scholar]
- 3.Clevers H. Modeling Development and Disease with Organoids. Cell. 2016;165:1586–97. [DOI] [PubMed] [Google Scholar]
- 4.Kapałczyńska M, Kolenda T, Przybyła W, Zajączkowska M, Teresiak A, Filas V, Ibbs M, Bliźniak R, Łuczewski Ł, Lamperska K. 2D and 3D cell cultures - a comparison of different types of cancer cell cultures. Arch Med Sci. 2018;14:910–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.HogenEsch H, Nikitin AY. Challenges in pre-clinical testing of anti-cancer drugs in cell culture and in animal models. J Control Release. 2012;164:183–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiklander OPB, Brennan M, Lötvall J, Breakefield XO. El Andaloussi S: advances in therapeutic applications of extracellular vesicles. Sci Transl Med. 2019;11:492. [DOI] [PMC free article] [PubMed]
- 7.Witwer KW, Goberdhan DC, O’Driscoll L, Théry C, Welsh JA, Blenkiron C, Buzás EI, Di Vizio D, Erdbrügger U, Falcón-Pérez JM, et al. Updating MISEV: evolving the minimal requirements for studies of extracellular vesicles. J Extracell Vesicles. 2021;10:e12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tominaga N, Kosaka N, Ono M, Katsuda T, Yoshioka Y, Tamura K, Lötvall J, Nakagama H, Ochiya T. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat Commun. 2015;6:6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bock C, Boutros M, Camp JG, Clarke L, Clevers H, Knoblich JA, Liberali P, Regev A, Rios AC, Stegle O, et al. The Organoid Cell Atlas. Nat Biotechnol. 2021;39:13–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garreta E, Kamm RD, Chuva de Sousa Lopes SM, Lancaster MA, Weiss R, Trepat X, Hyun I, Montserrat N. Rethinking organoid technology through bioengineering. Nat Mater. 2021;20:145–55. [DOI] [PubMed] [Google Scholar]
- 11.Brandenberg N, Hoehnel S, Kuttler F, Homicsko K, Ceroni C, Ringel T, Gjorevski N, Schwank G, Coukos G, Turcatti G, Lutolf MP. High-throughput automated organoid culture via stem-cell aggregation in microcavity arrays. Nat Biomed Eng. 2020;4:863–74. [DOI] [PubMed] [Google Scholar]
- 12.Wang Q, Guo F, Jin Y, Ma Y. Applications of human organoids in the personalized treatment for digestive diseases. Signal Transduct Target Ther. 2022;7:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drost J, Clevers H. Organoids in cancer research. Nat Rev Cancer. 2018;18:407–18. [DOI] [PubMed] [Google Scholar]
- 14.Corrò C, Novellasdemunt L, Li VSW. A brief history of organoids. Am J Physiol Cell Physiol. 2020;319:C151–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rauch I, Deets KA, Ji DX, von Moltke J, Tenthorey JL, Lee AY, Philip NH, Ayres JS, Brodsky IE, Gronert K, Vance RE. NAIP-NLRC4 inflammasomes coordinate intestinal epithelial cell expulsion with eicosanoid and IL-18 release via activation of Caspase-1 and – 8. Immunity. 2017;46:649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagar LE, Salahudeen A, Constantz CM, Wendel BS, Lyons MM, Mallajosyula V, Jatt LP, Adamska JZ, Blum LK, Gupta N, et al. Modeling human adaptive immune responses with tonsil organoids. Nat Med. 2021;27:125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huch M, Gehart H, van Boxtel R, Hamer K, Blokzijl F, Verstegen MM, Ellis E, van Wenum M, Fuchs SA, de Ligt J, et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015;160:299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141:1762–72. [DOI] [PubMed] [Google Scholar]
- 19.Di Lullo E, Kriegstein AR. The use of brain organoids to investigate neural development and disease. Nat Rev Neurosci. 2017;18:573–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shinozawa T, Kimura M, Cai Y, Saiki N, Yoneyama Y, Ouchi R, Koike H, Maezawa M, Zhang RR, Dunn A, et al. High-Fidelity Drug-Induced Liver Injury screen using human pluripotent stem cell-derived Organoids. Gastroenterology. 2021;160:831–e846810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu H, Uchimura K, Donnelly EL, Kirita Y, Morris SA, Humphreys BD. Comparative analysis and refinement of human PSC-Derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell. 2018;23:869–e881868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J, Koo BK, Knoblich JA. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. 2020;21:571–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Lopes SM, Little MH. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2016;536:238. [DOI] [PubMed] [Google Scholar]
- 24.Freedman BS, Brooks CR, Lam AQ, Fu H, Morizane R, Agrawal V, Saad AF, Li MK, Hughes MR, Werff RV, et al. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun. 2015;6:8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen S, Chen X, Geng Z, Su J. The horizon of bone organoid: a perspective on construction and application. Bioact Mater. 2022;18:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Wu Y, Li G, Zhou F, Wu X, Wang M, Liu X, Tang H, Bai L, Geng Z et al. Engineering large-scale self-mineralizing bone organoids with bone matrix-inspired Hydroxyapatite Hybrid Bioinks. Adv Mater 2024;36:2309875. [DOI] [PubMed]
- 27.Chen W, Zhang H, Zhou Q, Zhou F, Zhang Q, Su J. Smart Hydrogels for Bone Reconstruction via modulating the Microenvironment. Res (Wash D C). 2023;6:0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu Y, Zhang H, Wang S, Cao L, Zhou F, Jing Y, Su J. Bone/cartilage organoid on-chip: construction strategy and application. Bioact Mater. 2023;25:29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H, Wang L, Cui J, Wang S, Han Y, Shao H, Wang C, Hu Y, Li X, Zhou Q, et al. Maintaining hypoxia environment of subchondral bone alleviates osteoarthritis progression. Sci Adv. 2023;9:eabo7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Man K, Eisenstein NM, Hoey DA, Cox SC. Bioengineering extracellular vesicles: smart nanomaterials for bone regeneration. J Nanobiotechnol. 2023;21:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu Y, Chen X, Wang S, Jing Y, Su J. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 2021;9:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xue X, Hu Y, Wang S, Chen X, Jiang Y, Su J. Fabrication of physical and chemical crosslinked hydrogels for bone tissue engineering. Bioact Mater. 2022;12:327–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao Q, Wang L, Wang S, Huang B, Jing Y, Su J. Bone marrow mesenchymal stromal cells: identification, classification, and differentiation. Front Cell Dev Biol. 2021;9:787118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie C, Liang R, Ye J, Peng Z, Sun H, Zhu Q, Shen X, Hong Y, Wu H, Sun W, et al. High-efficient engineering of osteo-callus organoids for rapid bone regeneration within one month. Biomaterials. 2022;288:121741. [DOI] [PubMed] [Google Scholar]
- 35.Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X, Ichinose S, Nagaishi T, Okamoto R, Tsuchiya K, et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5⁺ stem cell. Nat Med. 2012;18:618–23. [DOI] [PubMed] [Google Scholar]
- 36.Jgamadze D, Lim JT, Zhang Z, Harary PM, Germi J, Mensah-Brown K, Adam CD, Mirzakhalili E, Singh S, Gu JB, et al. Structural and functional integration of human forebrain organoids with the injured adult rat visual system. Cell Stem Cell. 2023;30:137–e152137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sampaziotis F, Muraro D, Tysoe OC, Sawiak S, Beach TE, Godfrey EM, Upponi SS, Brevini T, Wesley BT, Garcia-Bernardo J, et al. Cholangiocyte organoids can repair bile ducts after transplantation in the human liver. Science. 2021;371:839–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neurath M. Current and emerging therapeutic targets for IBD. Nat Rev Gastroenterol Hepatol. 2017;14:688. [DOI] [PubMed] [Google Scholar]
- 39.Guo J, Wang F, Hu Y, Luo Y, Wei Y, Xu K, Zhang H, Liu H, Bo L, Lv S, et al. Exosome-based bone-targeting drug delivery alleviates impaired osteoblastic bone formation and bone loss in inflammatory bowel diseases. Cell Rep Med. 2023;4:100881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okamoto R, Mizutani T, Shimizu H. Development and application of Regenerative Medicine in Inflammatory Bowel Disease. Digestion. 2023;104:24–9. [DOI] [PubMed] [Google Scholar]
- 41.Dancause N, Barbay S, Frost SB, Plautz EJ, Chen D, Zoubina EV, Stowe AM, Nudo RJ. Extensive cortical rewiring after brain injury. J Neurosci. 2005;25:10167–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kempermann G, Gage FH, Aigner L, Song H, Curtis MA, Thuret S, Kuhn HG, Jessberger S, Frankland PW, Cameron HA, et al. Human adult neurogenesis: evidence and remaining questions. Cell Stem Cell. 2018;23:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: a public health perspective. J Head Trauma Rehabil. 1999;14:602–15. [DOI] [PubMed] [Google Scholar]
- 44.Gaillard A, Prestoz L, Dumartin B, Cantereau A, Morel F, Roger M, Jaber M. Reestablishment of damaged adult motor pathways by grafted embryonic cortical neurons. Nat Neurosci. 2007;10:1294–9. [DOI] [PubMed] [Google Scholar]
- 45.Girman SV, Golovina IL. Electrophysiological properties of embryonic neocortex transplants replacing the primary visual cortex of adult rats. Brain Res. 1990;523:78–86. [DOI] [PubMed] [Google Scholar]
- 46.Kadoshima T, Sakaguchi H, Nakano T, Soen M, Ando S, Eiraku M, Sasai Y. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc Natl Acad Sci U S A. 2013;110:20284–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paşca AM, Sloan SA, Clarke LE, Tian Y, Makinson CD, Huber N, Kim CH, Park JY, O’Rourke NA, Nguyen KD, et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods. 2015;12:671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, et al. Brain-region-specific Organoids using mini-bioreactors for modeling ZIKV exposure. Cell. 2016;165:1238–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qian X, Su Y, Adam CD, Deutschmann AU, Pather SR, Goldberg EM, Su K, Li S, Lu L, Jacob F, et al. Sliced human cortical organoids for modeling distinct cortical layer formation. Cell Stem Cell. 2020;26:766–e781769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tysoe OC, Justin AW, Brevini T, Chen SE, Mahbubani KT, Frank AK, Zedira H, Melum E, Saeb-Parsy K, Markaki AE, et al. Isolation and propagation of primary human cholangiocyte organoids for the generation of bioengineered biliary tissue. Nat Protoc. 2019;14:1884–925. [DOI] [PubMed] [Google Scholar]
- 51.Sampaziotis F, Justin AW, Tysoe OC, Sawiak S, Godfrey EM, Upponi SS, Gieseck RL 3rd, de Brito MC, Berntsen NL, Gómez-Vázquez MJ, et al. Reconstruction of the mouse extrahepatic biliary tree using primary human extrahepatic cholangiocyte organoids. Nat Med. 2017;23:954–63. [DOI] [PubMed] [Google Scholar]
- 52.Ouchi R, Togo S, Kimura M, Shinozawa T, Koido M, Koike H, Thompson W, Karns RA, Mayhew CN, McGrath PS, et al. Modeling steatohepatitis in humans with pluripotent stem cell-derived Organoids. Cell Metab. 2019;30:374–e384376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giandomenico SL, Sutcliffe M, Lancaster MA. Generation and long-term culture of advanced cerebral organoids for studying later stages of neural development. Nat Protoc. 2021;16:579–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J, Li X, Wang S, Cui J, Ren X, Su J. Bone-targeted exosomes: strategies and applications. Adv Healthc Mater. 2023;12:2203361. [DOI] [PubMed]
- 55.Song H, Li X, Zhao Z, Qian J, Wang Y, Cui J, Weng W, Cao L, Chen X, Hu Y, Su J. Reversal of osteoporotic activity by endothelial cell-secreted bone targeting and biocompatible exosomes. Nano Lett. 2019;19:3040–8. [DOI] [PubMed] [Google Scholar]
- 56.Zhu Z, Hu E, Shen H, Tan J, Zeng S. The functional and clinical roles of liquid biopsy in patient-derived models. J Hematol Oncol. 2023;16:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zaborowski MP, Balaj L, Breakefield XO, Lai CP. Extracellular vesicles: composition, Biological relevance, and methods of study. Bioscience. 2015;65:783–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zha QB, Yao YF, Ren ZJ, Li XJ, Tang JH. Extracellular vesicles: an overview of biogenesis, function, and role in breast cancer. Tumour Biol. 2017;39:1010428317691182. [DOI] [PubMed] [Google Scholar]
- 60.Bashyal S, Thapa C, Lee S. Recent progresses in exosome-based systems for targeted drug delivery to the brain. J Control Release. 2022;348:723–44. [DOI] [PubMed] [Google Scholar]
- 61.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–28. [DOI] [PubMed] [Google Scholar]
- 62.Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9–17. [DOI] [PubMed] [Google Scholar]
- 63.Maas SLN, Breakefield XO, Weaver AM. Extracellular vesicles: Unique Intercellular Delivery vehicles. Trends Cell Biol. 2017;27:172–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chivet M, Javalet C, Laulagnier K, Blot B, Hemming FJ, Sadoul R. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J Extracell Vesicles. 2014;3:24722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM, Baty CJ, Gibson GA, Erdos G, Wang Z, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119:756–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Record M, Carayon K, Poirot M, Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta. 2014;1841:108–20. [DOI] [PubMed] [Google Scholar]
- 67.Nguyen A, Yaffe MB. Proteomics and systems biology approaches to signal transduction in sepsis. Crit Care Med. 2003;31:S1–6. [DOI] [PubMed] [Google Scholar]
- 68.Gonda A, Kabagwira J, Senthil GN, Wall NR. Internalization of exosomes through receptor-mediated endocytosis. Mol Cancer Res. 2019;17:337–47. [DOI] [PubMed] [Google Scholar]
- 69.Murphy DE, de Jong OG, Brouwer M, Wood MJ, Lavieu G, Schiffelers RM, Vader P. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp Mol Med. 2019;51:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Q, Wang L, Wang S, Cheng H, Xu L, Pei G, Wang Y, Fu C, Jiang Y, He C, Wei Q. Signaling pathways and targeted therapy for myocardial infarction. Signal Transduct Target Ther. 2022;7:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhong L, Liao D, Li J, Liu W, Wang J, Zeng C, Wang X, Cao Z, Zhang R, Li M, et al. Rab22a-NeoF1 fusion protein promotes osteosarcoma lung metastasis through its secretion into exosomes. Signal Transduct Target Ther. 2021;6:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kimiz-Gebologlu I, Oncel SS. Exosomes: large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J Control Release. 2022;347:533–43. [DOI] [PubMed] [Google Scholar]
- 73.Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu H, Zhang H, Han Y, Hu Y, Geng Z, Su J. Bacterial extracellular vesicles-based therapeutic strategies for bone and soft tissue tumors therapy. Theranostics. 2022;12:6576–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Y, Lin Q, Zhang H, Wang S, Cui J, Hu Y, Liu J, Li M, Zhang K, Zhou F, et al. M2 macrophage-derived exosomes promote diabetic fracture healing by acting as an immunomodulator. Bioact Mater. 2023;28:273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gu Y, Zhao J, Wang J, Jiang Y, Jing Y, Xu K, Su J. Osteoblast-derived extracellular vesicles exert bone formation effects by WIF1-mediated regulation of mitophagy. Med Plus. 2024;1:100033.
- 77.Gao H, Zeng Y, Huang X, Liang AL, Xie Q, Lin J, Gong X, Fan J, Zou X, Xu T. Extracellular vesicles from organoid-derived human retinal progenitor cells prevent lipid overload-induced retinal pigment epithelium injury by regulating fatty acid metabolism. J Extracell Vesicles. 2024;13:e12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hayashi T, Lombaert IM, Hauser BR, Patel VN, Hoffman MP. Exosomal MicroRNA Transport from Salivary Mesenchyme regulates epithelial progenitor expansion during Organogenesis. Dev Cell. 2017;40:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chansaenroj A, Adine C, Charoenlappanit S, Roytrakul S, Sariya L, Osathanon T, Rungarunlert S, Urkasemsin G, Chaisuparat R, Yodmuang S, et al. Magnetic bioassembly platforms towards the generation of extracellular vesicles from human salivary gland functional organoids for epithelial repair. Bioact Mater. 2022;18:151–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee H. New Technologies for Analysis of Extracellular Vesicles. Chem Rev. 2018;118:1917–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Q, Jeppesen DK, Higginbotham JN, Franklin JL, Coffey RJ. Comprehensive isolation of extracellular vesicles and nanoparticles. Nat Protoc. 2023;18:1462–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Iwai K, Minamisawa T, Suga K, Yajima Y, Shiba K. Isolation of human salivary extracellular vesicles by iodixanol density gradient ultracentrifugation and their characterizations. J Extracell Vesicles. 2016;5:30829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ikeda G, Santoso MR, Tada Y, Li AM, Vaskova E, Jung JH, O’Brien C, Egan E, Ye J, Yang PC. Mitochondria-Rich Extracellular vesicles from autologous stem cell-derived cardiomyocytes restore energetics of ischemic myocardium. J Am Coll Cardiol. 2021;77:1073–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kornilov R, Puhka M, Mannerström B, Hiidenmaa H, Peltoniemi H, Siljander P, Seppänen-Kaijansinkko R, Kaur S. Efficient ultrafiltration-based protocol to deplete extracellular vesicles from fetal bovine serum. J Extracell Vesicles. 2018;7:1422674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yasui T, Paisrisarn P, Yanagida T, Konakade Y, Nakamura Y, Nagashima K, Musa M, Thiodorus IA, Takahashi H, Naganawa T, et al. Molecular profiling of extracellular vesicles via charge-based capture using oxide nanowire microfluidics. Biosens Bioelectron. 2021;194:113589. [DOI] [PubMed] [Google Scholar]
- 86.Guo J, Wu C, Lin X, Zhou J, Zhang J, Zheng W, Wang T, Cui Y. Establishment of a simplified dichotomic size-exclusion chromatography for isolating extracellular vesicles toward clinical applications. J Extracell Vesicles. 2021;10:e12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yuan X, Sun L, Jeske R, Nkosi D, York SB, Liu Y, Grant SC, Meckes DG Jr., Li Y. Engineering extracellular vesicles by three-dimensional dynamic culture of human mesenchymal stem cells. J Extracell Vesicles. 2022;11:e12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jalilian E, Massoumi H, Bigit B, Amin S, Katz EA, Guaiquil VH, Anwar KN, Hematti P, Rosenblatt MI, Djalilian AR. Bone marrow mesenchymal stromal cells in a 3D system produce higher concentration of extracellular vesicles (EVs) with increased complexity and enhanced neuronal growth properties. Stem Cell Res Ther. 2022;13:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carter K, Lee HJ, Na KS, Fernandes-Cunha GM, Blanco IJ, Djalilian A, Myung D. Characterizing the impact of 2D and 3D culture conditions on the therapeutic effects of human mesenchymal stem cell secretome on corneal wound healing in vitro and ex vivo. Acta Biomater. 2019;99:247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rocha S, Carvalho J, Oliveira P, Voglstaetter M, Schvartz D, Thomsen AR, Walter N, Khanduri R, Sanchez JC, Keller A, et al. 3D Cellular Architecture affects MicroRNA and Protein Cargo of Extracellular vesicles. Adv Sci (Weinh). 2019;6:1800948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sun L, Ji Y, Chi B, Xiao T, Li C, Yan X, Xiong X, Mao L, Cai D, Zou A, et al. A 3D culture system improves the yield of MSCs-derived extracellular vesicles and enhances their therapeutic efficacy for heart repair. Biomed Pharmacother. 2023;161:114557. [DOI] [PubMed] [Google Scholar]
- 92.Yang L, Zhai Y, Hao Y, Zhu Z, Cheng G. The Regulatory functionality of Exosomes derived from hUMSCs in 3D culture for Alzheimer’s Disease Therapy. Small. 2020;16:e1906273. [DOI] [PubMed] [Google Scholar]
- 93.Zhang Y, Chopp M, Zhang ZG, Katakowski M, Xin H, Qu C, Ali M, Mahmood A, Xiong Y. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem Int. 2017;111:69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zinger A, Cvetkovic C, Sushnitha M, Naoi T, Baudo G, Anderson M, Shetty A, Basu N, Covello J, Tasciotti E, et al. Humanized biomimetic nanovesicles for Neuron Targeting. Adv Sci (Weinh). 2021;8:e2101437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Su Y, Sun X, Liu X, Qu Q, Yang L, Chen Q, Liu F, Li Y, Wang Q, Huang B, et al. hUC-EVs-ATO reduce the severity of acute GVHD by resetting inflammatory macrophages toward the M2 phenotype. J Hematol Oncol. 2022;15:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Upadhya R, Shetty AK. Extracellular vesicles for the diagnosis and treatment of Parkinson’s Disease. Aging Dis. 2021;12:1438–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang T, Dong Y, Wan J, Liu X, Liu Y, Huang J, Zhou J, Xiao H, Tang L, Wang Y et al. Sustained release of BMSC-EVs from 3D Printing Gel/HA/nHAP scaffolds for promoting bone regeneration in Diabetic rats. Adv Healthc Mater. 2023;12:2203131. [DOI] [PubMed]
- 98.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9. [DOI] [PubMed] [Google Scholar]
- 99.Théry C, Duban L, Segura E, Véron P, Lantz O, Amigorena S. Indirect activation of naïve CD4 + T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3:1156–62. [DOI] [PubMed] [Google Scholar]
- 100.Chen X, Song CH, Feng BS, Li TL, Li P, Zheng PY, Chen XM, Xing Z, Yang PC. Intestinal epithelial cell-derived integrin αβ6 plays an important role in the induction of regulatory T cells and inhibits an antigen-specific Th2 response. J Leukoc Biol. 2011;90:751–9. [DOI] [PubMed] [Google Scholar]
- 101.Pinto DO, Al Sharif S, Mensah G, Cowen M, Khatkar P, Erickson J, Branscome H, Lattanze T, DeMarino C, Alem F, et al. Extracellular vesicles from HTLV-1 infected cells modulate target cells and viral spread. Retrovirology. 2021;18:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roush S, Slack FJ. The let-7 family of microRNAs. Trends Cell Biol. 2008;18:505–16. [DOI] [PubMed] [Google Scholar]
- 103.Lee H, Han S, Kwon CS, Lee D. Biogenesis and regulation of the let-7 miRNAs and their functional implications. Protein Cell. 2016;7:100–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang Y, Yan Y, Meng J, Girotra M, Ramakrishnan S, Roy S. Immune modulation mediated by extracellular vesicles of intestinal organoids is disrupted by opioids. Mucosal Immunol. 2021;14:887–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Meyer-Franke A, Kaplan MR, Pfrieger FW, Barres BA. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15:805–19. [DOI] [PubMed] [Google Scholar]
- 106.Eastlake K, Lamb WDB, Luis J, Khaw PT, Jayaram H, Limb GA. Prospects for the application of Müller glia and their derivatives in retinal regenerative therapies. Prog Retin Eye Res. 2021;85:100970. [DOI] [PubMed] [Google Scholar]
- 107.Limb GA, Salt TE, Munro PM, Moss SE, Khaw PT. In vitro characterization of a spontaneously immortalized human Müller cell line (MIO-M1). Invest Ophthalmol Vis Sci. 2002;43:864–9. [PubMed] [Google Scholar]
- 108.Bonilla-Pons S, Nakagawa S, Bahima EG, Fernández-Blanco Á, Pesaresi M, D’Antin JC, Sebastian-Perez R, Greco D, Domínguez-Sala E, Gómez-Riera R, et al. Müller glia fused with adult stem cells undergo neural differentiation in human retinal models. EBioMedicine. 2022;77:103914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Eastlake K, Wang W, Jayaram H, Murray-Dunning C, Carr AJF, Ramsden CM, Vugler A, Gore K, Clemo N, Stewart M, et al. Phenotypic and functional characterization of Müller Glia isolated from Induced Pluripotent Stem Cell-Derived Retinal organoids: improvement of retinal ganglion cell function upon transplantation. Stem Cells Transl Med. 2019;8:775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zou T, Gao L, Zeng Y, Li Q, Li Y, Chen S, Hu X, Chen X, Fu C, Xu H, Yin ZQ. Organoid-derived C-Kit(+)/SSEA4(-) human retinal progenitor cells promote a protective retinal microenvironment during transplantation in rodents. Nat Commun. 2019;10:1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Singh MS, Park SS, Albini TA, Canto-Soler MV, Klassen H, MacLaren RE, Takahashi M, Nagiel A, Schwartz SD, Bharti K. Retinal stem cell transplantation: balancing safety and potential. Prog Retin Eye Res. 2020;75:100779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bian B, Zhao C, He X, Gong Y, Ren C, Ge L, Zeng Y, Li Q, Chen M, Weng C, et al. Exosomes derived from neural progenitor cells preserve photoreceptors during retinal degeneration by inactivating microglia. J Extracell Vesicles. 2020;9:1748931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Arthur P, Kandoi S, Sun L, Kalvala A, Kutlehria S, Bhattacharya S, Kulkarni T, Nimma R, Li Y, Lamba DA, Singh M. Biophysical, Molecular and Proteomic Profiling of Human Retinal Organoid-Derived exosomes. Pharm Res 2022;40:801-16. [DOI] [PMC free article] [PubMed]
- 114.Zhou J, Flores-Bellver M, Pan J, Benito-Martin A, Shi C, Onwumere O, Mighty J, Qian J, Zhong X, Hogue T, et al. Human retinal organoids release extracellular vesicles that regulate gene expression in target human retinal progenitor cells. Sci Rep. 2021;11:21128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang S, Poli S, Liang X, Peng GH. Longitudinal single-cell RNA-seq of hESCs-derived retinal organoids. Sci China Life Sci. 2021;64:1661–76. [DOI] [PubMed] [Google Scholar]
- 116.He C, Zheng S, Luo Y, Wang B. Exosome Theranostics: Biology and Translational Medicine. Theranostics. 2018;8:237–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Arthur P, Muok L, Nathani A, Zeng EZ, Sun L, Li Y, Singh M. Bioengineering Human pluripotent stem cell-derived retinal organoids and Optic Vesicle-containing brain Organoids for Ocular diseases. Cells 2022;11:11213429. [DOI] [PMC free article] [PubMed]
- 118.Kwak S, Song CL, Lee J, Kim S, Nam S, Park YJ, Lee J. Development of pluripotent stem cell-derived epidermal organoids that generate effective extracellular vesicles in skin regeneration. Biomaterials. 2024;307:122522. [DOI] [PubMed] [Google Scholar]
- 119.Jiang Y, Li J, Xue X, Yin Z, Xu K, Su J. Engineered extracellular vesicles for bone therapy. Nano Today. 2022;44:101487.
- 120.Zhang H, Wu S, Chen W, Hu Y, Geng Z, Su J. Bone/cartilage targeted hydrogel: strategies and applications. Bioact Mater. 2023;23:156–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu H, Song P, Zhang H, Zhou F, Ji N, Wang M, Zhou G, Han R, Liu X, Weng W, et al. Synthetic biology-based bacterial extracellular vesicles displaying BMP-2 and CXCR4 to ameliorate osteoporosis. J Extracell Vesicles. 2024;13:e12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lin Y, Wu J, Gu W, Huang Y, Tong Z, Huang L, Tan J. Exosome-liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Adv Sci (Weinh). 2018;5:1700611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hao Z, Ren L, Zhang Z, Yang Z, Wu S, Liu G, Cheng B, Wu J, Xia J. A multifunctional neuromodulation platform utilizing Schwann cell-derived exosomes orchestrates bone microenvironment via immunomodulation, angiogenesis and osteogenesis. Bioact Mater. 2023;23:206–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Smyth T, Petrova K, Payton NM, Persaud I, Redzic JS, Graner MW, Smith-Jones P, Anchordoquy TJ. Surface functionalization of exosomes using click chemistry. Bioconjug Chem. 2014;25:1777–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cao Y, Wu T, Zhang K, Meng X, Dai W, Wang D, Dong H, Zhang X. Engineered exosome-mediated Near-Infrared-II region V(2)C Quantum dot delivery for Nucleus-Target Low-Temperature Photothermal Therapy. ACS Nano. 2019;13:1499–510. [DOI] [PubMed] [Google Scholar]
- 126.Piffoux M, Silva AKA, Wilhelm C, Gazeau F, Tareste D. Modification of Extracellular vesicles by Fusion with liposomes for the design of Personalized Biogenic Drug Delivery systems. ACS Nano. 2018;12:6830–42. [DOI] [PubMed] [Google Scholar]
- 127.Mi B, Chen L, Xiong Y, Yang Y, Panayi AC, Xue H, Hu Y, Yan C, Hu L, Xie X, et al. Osteoblast/Osteoclast and Immune Cocktail Therapy of an Exosome/Drug delivery multifunctional hydrogel accelerates fracture repair. ACS Nano. 2022;16:771–82. [DOI] [PubMed] [Google Scholar]
- 128.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–5. [DOI] [PubMed] [Google Scholar]
- 129.Zha Y, Li Y, Lin T, Chen J, Zhang S, Wang J. Progenitor cell-derived exosomes endowed with VEGF plasmids enhance osteogenic induction and vascular remodeling in large segmental bone defects. Theranostics. 2021;11:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lin Z, Xiong Y, Meng W, Hu Y, Chen L, Chen L, Xue H, Panayi AC, Zhou W, Sun Y, et al. Exosomal PD-L1 induces osteogenic differentiation and promotes fracture healing by acting as an immunosuppressant. Bioact Mater. 2022;13:300–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Manhas J, Edelstein HI, Leonard JN, Morsut L. The evolution of synthetic receptor systems. Nat Chem Biol. 2022;18:244–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang ZJ, Yu ZY, Cai YM, Du RR, Cai L. Engineering of an enhanced synthetic notch receptor by reducing ligand-independent activation. Commun Biol. 2020;3:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Trentesaux C, Yamada T, Klein OD, Lim WA. Harnessing synthetic biology to engineer organoids and tissues. Cell Stem Cell. 2023;30:10–9. [DOI] [PubMed] [Google Scholar]
- 134.Yi K, Rong Y, Huang L, Tang X, Zhang Q, Wang W, Wu J, Wang F. Aptamer-Exosomes for Tumor Theranostics. ACS Sens. 2021;6:1418–29. [DOI] [PubMed] [Google Scholar]
- 135.Sun S, Liu H, Hu Y, Wang Y, Zhao M, Yuan Y, Han Y, Jing Y, Cui J, Ren X, et al. Selection and identification of a novel ssDNA aptamer targeting human skeletal muscle. Bioact Mater. 2023;20:166–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cui Y, Guo Y, Kong L, Shi J, Liu P, Li R, Geng Y, Gao W, Zhang Z, Fu D. A bone-targeted engineered exosome platform delivering siRNA to treat osteoporosis. Bioact Mater. 2022;10:207–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu H, Li M, Zhang T, Liu X, Zhang H, Geng Z, Su J. Engineered bacterial extracellular vesicles for osteoporosis therapy. Chem Eng J. 2022;450:138309.
- 138.Yang Y, Hong Y, Nam GH, Chung JH, Koh E, Kim IS. Virus-mimetic fusogenic exosomes for direct delivery of Integral Membrane Proteins to target cell membranes. Adv Mater. 2017;29:1605604. [DOI] [PubMed]
- 139.Chatterjee M, Özdemir S, Kunadt M, Koel-Simmelink M, Boiten W, Piepkorn L, Pham TV, Chiasserini D, Piersma SR, Knol JC et al. C1q is increased in cerebrospinal fluid-derived extracellular vesicles in Alzheimer’s disease: A multi-cohort proteomics and immuno-assay validation study. Alzheimers Dement. 2023;19:4828–40. [DOI] [PubMed]
- 140.Lee J, Lee H, Goh U, Kim J, Jeong M, Lee J, Park JH. Cellular Engineering with membrane Fusogenic liposomes to produce Functionalized Extracellular vesicles. ACS Appl Mater Interfaces. 2016;8:6790–5. [DOI] [PubMed] [Google Scholar]
- 141.Xiong Y, Mi BB, Lin Z, Hu YQ, Yu L, Zha KK, Panayi AC, Yu T, Chen L, Liu ZP, et al. The role of the immune microenvironment in bone, cartilage, and soft tissue regeneration: from mechanism to therapeutic opportunity. Mil Med Res. 2022;9:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xiong Y, Chen L, Liu P, Yu T, Lin C, Yan C, Hu Y, Zhou W, Sun Y, Panayi AC, et al. All-in-One: multifunctional hydrogel accelerates oxidative Diabetic Wound Healing through timed-release of exosome and fibroblast growth factor. Small. 2022;18:e2104229. [DOI] [PubMed] [Google Scholar]
- 143.Liu H, Zhang H, Wang S, Cui J, Weng W, Liu X, Tang H, Hu Y, Li X, Zhang K et al. Bone-targeted bioengineered bacterial extracellular vesicles delivering siRNA to ameliorate osteoporosis. Compos B Eng. 2023;255:110610.
- 144.Rossi G, Manfrin A, Lutolf MP. Progress and potential in organoid research. Nat Rev Genet. 2018;19:671–87. [DOI] [PubMed] [Google Scholar]
- 145.Fortunato D, Giannoukakos S, Giménez-Capitán A, Hackenberg M, Molina-Vila MA, Zarovni N. Selective isolation of extracellular vesicles from minimally processed human plasma as a translational strategy for liquid biopsies. Biomark Res. 2022;10:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yang R, Yu Y. Patient-derived organoids in translational oncology and drug screening. Cancer Lett. 2023;562:216180. [DOI] [PubMed] [Google Scholar]
- 147.Wang Y, Jeon H. 3D cell cultures toward quantitative high-throughput drug screening. Trends Pharmacol Sci. 2022;43:569–81. [DOI] [PubMed] [Google Scholar]
- 148.Ramezankhani R, Solhi R, Chai YC, Vosough M, Verfaillie C. Organoid and microfluidics-based platforms for drug screening in COVID-19. Drug Discov Today. 2022;27:1062–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Geyer M, Queiroz K. Microfluidic platforms for high-throughput pancreatic ductal adenocarcinoma Organoid Culture and Drug Screening. Front Cell Dev Biol. 2021;9:761807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Geng Z, Sang S, Wang S, Meng F, Li Z, Zhu S, Cui Z, Jing Y, Wang C, Su J. Optimizing the strontium content to achieve an ideal osseointegration through balancing apatite-forming ability and osteogenic activity. Biomater Adv. 2022;133:112647. [DOI] [PubMed] [Google Scholar]
- 151.Wu S, Wu X, Wang X, Su J. Hydrogels for bone organoid construction: from a materiobiological perspective. J Mater Sci Technol. 2023;136:21–31. [Google Scholar]
- 152.Yi SA, Zhang Y, Rathnam C, Pongkulapa T, Lee KB. Bioengineering approaches for the Advanced Organoid Research. Adv Mater. 2021;33:e2007949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bouffi C, Wikenheiser-Brokamp KA, Chaturvedi P, Sundaram N, Goddard GR, Wunderlich M, Brown NE, Staab JF, Latanich R, Zachos NC et al. In vivo development of immune tissue in human intestinal organoids transplanted into humanized mice. Nat Biotechnol. 2023;41:824–31. [DOI] [PMC free article] [PubMed]
- 154.Workman MJ, Mahe MM, Trisno S, Poling HM, Watson CL, Sundaram N, Chang CF, Schiesser J, Aubert P, Stanley EG, et al. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat Med. 2017;23:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu H, Su J. Organoid and organoid extracellular vesicles for osteoporotic fractures therapy: current status and future perspectives. IMed. 2023;1:e20230011.
- 156.Liu H, Zhang Q, Wang S, Weng W, Jing Y, Su J. Bacterial extracellular vesicles as bioactive nanocarriers for drug delivery: advances and perspectives. Bioact Mater. 2022;14:169–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu H, Geng Z, Su J. Engineered mammalian and bacterial extracellular vesicles as promising nanocarriers for targeted therapy. EVCNA. 2022;3:63–86. [Google Scholar]
- 158.Ji N, Wang F, Wang M, Zhang W, Liu H, Su J. Engineered bacterial extracellular vesicles for central nervous system diseases. J Control Release. 2023;364:46–60. [DOI] [PubMed] [Google Scholar]
- 159.Rutter BD, Innes RW. Extracellular vesicles isolated from the Leaf Apoplast carry stress-response proteins. Plant Physiol. 2017;173:728–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nemati M, Singh B, Mir RA, Nemati M, Babaei A, Ahmadi M, Rasmi Y, Golezani AG, Rezaie J. Plant-derived extracellular vesicles: a novel nanomedicine approach with advantages and challenges. Cell Commun Signal. 2022;20:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Han R, Wu Y, Han Y, Liu X, Liu H, Su J. Engineered plant extracellular vesicles for autoimmune diseases therapy. Nano Res. 2023;17:2857–73.
- 162.Liu H, Sun J, Wang M, Wang S, Su J, Xu C. Intestinal organoids and organoids extracellular vesicles for inflammatory bowel disease treatment. Chem Eng J. 2023;465:142842.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.