Abstract
BACKGROUND:
Acinic cell carcinoma (AcCC) is diagnostically challenging on fine-needle aspiration because it can mimic several other neoplasms or even normal acinar tissue. Immunopositivity for DOG1, especially circumferential membranous staining, can support the diagnosis of AcCC but is not entirely specific, and it is prone to technical and interpretive challenges on small specimens. NR4A3 (nuclear receptor subfamily 4 group A member 3) translocation and nuclear NR4A3 overexpression were recently described in the majority of AcCCs. Here, the authors evaluate the performance of NR4A3 immunohistochemistry (IHC) and NR4A3 break-apart fluorescence in situ hybridization (FISH) on cell block preparations and compare them with DOG1 IHC in distinguishing AcCC from other entities in the differential diagnosis.
METHODS:
The authors identified 34 cytology cell blocks with lesional cells, including 11 specimens of AcCC (2 of which derived from 1 patient and showed high-grade transformation) as well as 2 secretory carcinomas, 7 salivary duct carcinomas, 4 mucoepidermoid carcinomas, 3 oncocytomas, 3 renal cell carcinomas, and 6 specimens containing nonneoplastic salivary gland tissue. NR4A3 IHC, DOG1 IHC, and NR4A3 FISH were attempted for all cases.
RESULTS:
NR4A3 IHC had 81.8% sensitivity and 100% specificity for AcCC, whereas NR4A3 FISH had 36.4% sensitivity and 100% specificity, although 4 cases (3 mucoepidermoid carcinomas and 1 salivary gland tissue sample) could not be analyzed because of low cellularity. Notably, no normal acinar tissue specimens showed NR4A3 positivity by IHC or FISH. In addition, DOG1 IHC had 72.7% sensitivity and 92% specificity.
CONCLUSIONS:
NR4A3 IHC is highly specific for the diagnosis of AcCC and is more sensitive than DOG1 IHC and NR4A3 FISH. In addition, NR4A3 IHC performance is not improved by the inclusion of DOG1 IHC. Finally, NR4A3 positivity resolves the perennial problem of distinguishing AcCC from normal acinar tissue.
Keywords: acinic cell carcinoma, cytology, DOG1, nuclear receptor subfamily 4 group A member 3 (NR4A3), salivary gland
INTRODUCTION
Acinic cell carcinoma (AcCC) is the second most common malignant neoplasm of the salivary glands, is located most commonly in the parotid gland, and derives from the acinar epithelium.1 The diagnosis of AcCC by fine-needle aspiration (FNA) is often challenging. First, AcCC may appear similar to other benign and malignant neoplasms with oncocytic, vacuolated, or clear cell features, including oncocytoma, secretory carcinoma (SC), salivary duct carcinoma (SDC), mucoepidermoid carcinoma (MEC), and metastatic renal cell carcinoma (RCC). Second, AcCC may be difficult to distinguish from nonneoplastic salivary gland tissue (SGT) by FNA because tumor cells are often low-grade and may not differ significantly in appearance from normal acinar cells. Although the malignant acinar cells in cytologic specimens of AcCC typically show a dispersed pattern instead of the lobular pattern characteristic of normal acini, definite distinction of these patterns may be difficult.2–4 Also, because AcCC may undergo high-grade transformation, it occasionally can mimic other high-grade salivary carcinomas.1–4
The difficulty of diagnosing AcCC by FNA is reflected in the Milan system. The category salivary gland neoplasm of uncertain malignant potential pertains to instances in which the cytologic features are diagnostic of a neoplasm but for which definite classification as benign or malignant is not possible. Within the category salivary gland neoplasm of uncertain malignant potential, the subcategory cellular oncocytic/oncocytoid neoplasm refers to cellular specimens with oncocytic cells lacking high-grade features, such as marked nuclear atypia, high mitotic activity, and necrosis. This subcategory includes AcCC and most of its morphologic mimics. In other instances, AcCC may be diagnosed as atypia of undetermined significance or suspicious for malignancy when a definitive diagnosis of neoplasia cannot be rendered, often because of the difficulty in distinguishing normal acini from AcCC.3
In salivary gland cytology, immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) are often used to aid diagnosis.3,5,6 DOG1 and, to a lesser extent, SOX-10 have been the primary markers of AcCC, although neither is entirely sensitive or specific.7–14 Also, AcCC is typically negative for p40 and p63 by IHC.12,14 DOG1 is primarily a marker of acinar differentiation, although intercalated ducts sometimes show weak staining. AcCC typically shows strong positivity for DOG1, appearing as a mixture of apical membranous, cytoplasmic, and complete membranous staining. Strong, complete membranous staining in particular is specific for AcCC. However, DOG1 also shows variably intense apical membranous staining in normal acini, which can complicate interpretation.10–13 DOG1 is also frequently positive in adenoid cystic carcinoma and epithelial-myoepithelial carcinoma and is sometimes positive in pleomorphic adenoma, polymorphous adenocarcinoma, carcinoma ex-pleomorphic adenoma, and mucoepidermoid carcinoma.10,13 Although SOX-10 is positive in AcCC, it is also positive in myoepithelioma, epithelial-myoepithelial carcinoma, pleomorphic adenoma, adenoid cystic carcinoma, SC, low-grade SDC, sialoblastoma, basal cell adenomas and carcinomas,7–9 and a subset of mucoepidermoid carcinoma.8,15 Overall, a set of IHC markers may assist in the cytologic diagnosis of AcCC, but none of these markers are entirely sensitive and specific, nor do they target a diagnostic molecular alteration.
Although IHC can be helpful in the workup of a salivary gland tumor, several salivary gland neoplasms show diagnostic translocations. These include ETV6 in SC, MAML2 in MEC, MYB in adenoid cystic carcinoma, EWSR1 in hyalinizing clear cell carcinoma, and PLAG1 or HMGA2 in pleomorphic adenoma (PA) and in carcinomas arising from PA.16–31 Recently, a recurrent (t[4;9][q13;q31]) genomic rearrangement was discovered in AcCC.32,33 In most AcCCs, this rearrangement causes translocation of active enhancer regions from the secretory Ca-binding phosphoprotein (SCPP) gene cluster to the region upstream of nuclear receptor subfamily 4 group A member 3 (NR4A3),33,34 leading to NR4A3 IHC overexpression.34–36 In a minority of AcCCs, the rearrangement instead leads to a gene fusion between Histatin 3 (HTN3) and Myb/SANT-like DNA-binding domain containing 3 (MSANTD3).32,34,37 Importantly, AcCCs with HTN3-MSANTD3 fusion also consistently overexpress NR4A3 by IHC.34
Both NR4A3 translocation (detectable by NR4A3 break-apart FISH), and nuclear NR4A3 overexpression (detectable by IHC), have been shown to be sensitive and specific for the diagnosis of AcCC in surgical resection specimens.33,34 However, because NR4A3 is also overexpressed in AcCCs that lack SCPP-NR4A3 translocation, NR4A3 IHC is thought to be more sensitive for AcCC than NR4A3 FISH.
In the current study, the performance of NR4A3 IHC, NR4A3 FISH, and DOG1 IHC on cytology cell block preparations was evaluated for the diagnosis of AcCC and for the distinction of AcCC from its morphologic mimics.
MATERIALS AND METHODS
This study was performed under Institutional Review Board approval. A search of the laboratory information system was conducted to identify potential cytology cases of AcCC, SC, SDC, MEC, oncocytoma, RCC, and SGT from 2007 through 2019. In total, 61 potential cases were identified, with 9 cases excluded because of an absence of cell block preparations, 7 excluded because of unavailable cell blocks, and 11 excluded because of absent lesional cells in the cell block preparations. The final 34 cases included 11 AcCCs, 2 SCs, 7 SDCs, 4 MECs, 3 oncocytomas, 3 RCCs, and 6 cases of nonneoplastic SGT. All cases were FNA specimens except for 1, which was a pleural fluid (MEC). All but 2 (AcCC and RCC) of the neoplastic cases had in-house surgical specimen confirmation of the diagnosis from the sampled site or a previous primary site. Of note, 2 cases of AcCC (1 primary, 1 lymph node metastasis) were from the same patient, and the surgical resection showed high-grade transformation. NR4A3 IHC and NR4A3 FISH were done on all 34 cytology cases and on 8 AcCC resection specimens. MSANTD3 FISH was also done on the 8 AcCC resection specimens.
Cell Block Preparation
The specimens were centrifuged in a 50-mL conical tube to form a pellet. The supernatant was poured off, and from 1 to 3 drops of Histogel (Richard Allan Scientific) were added with a disposable pipette. After the Histogel solidified, the pellet was transferred to a cassette, which was submitted for formalin fixation and paraffin-embedded tissue processing.
Immunohistochemistry
IHC for DOG1 (1:50 dilution; RM-9132-5 [1.1]; Thermo Fisher Scientific) was performed using an automated IHC system (Ventana BenchMark ULTRA; Roche Diagnostics) on 4-μm-thick, formalin-fixed, paraffin-embedded (FFPE) cell block sections. IHC for NR4A3 (1:50 dilution; SC-393902 [H-7]; Santa Cruz Biotechnology, Inc) was performed using an automated IHC system (Leica Bond-III; Leica Biosystems) on 4-μm-thick, FFPE cell block sections.
Staining was semiquantitatively scored for intensity (0, 1+, 2+, 3+) and extent (<1%, 1%−25%, 26%−50%, 51%−75%, 76%−100%) for both NR4A3 and DOG1. Staining of any intensity in at least 1% of cells was considered positive. For DOG1, staining was further classified based on its distribution as membranous (apical or complete) and/or cytoplasmic. Also, a secondary threshold of complete membranous staining for true DOG1 positivity was applied based on previous literature.10,12
Fluorescence In Situ Hybridization
NR4A3 FISH was done using a ZytoLight SPEC NR4A3 dual-color break-apart probe (1:10 dilution; Z-2145–50; ZytoVision). MSANTD3 FISH was done using an MSANTD3 break-apart probe (1:10 dilution; Empire Genomics). Blank 4-μm FFPE cell block sections were deparaffinized, dehydrated, and pretreated with 1 M sodium thiocyanate. Sections were then treated with pepsin for protein digestion and fixed in formalin. Denaturing was performed with 70% formamide at 75 °C. Probes were hybridized using an automated instrument (Dako Cytomation hybridizer; Dako A/S) and subsequently counterstained with DAPI. Target areas were marked by a pathologist, and cells were analyzed using BioView System (BioView) and Applied Imaging Cytovision Workstation (Applied Imaging, now under Leica Biosystems). At least 30 cells were analyzed for each case, with a target of 60 total cells if possible. A threshold of 20% was used for a positive NR4A3 result, and a threshold of 7% was used for MSANTD3 based on in-house validation.
RESULTS
The overall clinicopathologic features are listed in Table 1. Of the neoplastic salivary gland cases, 18 were located in a salivary gland (8 AcCCs, 4 SDCs, 3 MECs, 3 oncocytomas), 7 were located in a lymph node (3 AcCCs, 2 SCs, 2 SDCs), and 2 were located in other metastatic sites (1 SDC [pleural fluid], 1 MEC [lung]). All RCC cases were metastatic (1 each to the lung, chest wall, and neck lymph nodes). Nine (81.8%) of the AcCC cases had subsequent surgical resection material available.
TABLE 1.
Overview of Clinicopathologic Features
| Diagnosis | Case No. | No. of Men (%) | Age: Median [Range], y | Location: No. (%) | ||
|---|---|---|---|---|---|---|
| Salivary Gland | Lymph Node | Other | ||||
| Acinic cell carcinoma | 11 | 7 (63.6) | 66 [39–77] | 8 (72.7) | 3 (27.3) | 0 (0.0) |
| Neoplasm, not acinic cell carcinoma | 19 | 9 (47.4) | 68 [48–89] | 10 (52.6) | 5 (26.3) | 4 (21.1) |
| Oncocytoma | 3 | 0 (0.0) | 68 [57–84] | 3 (100) | 0 (0.0) | 0 (0.0) |
| Secretory carcinoma | 2 | 1 (50.0) | 60 [48–72] | 0 (0.0) | 2 (100) | 0 (0.0) |
| Salivary duct carcinoma | 7 | 4 (57.1) | 73 [66–89] | 4 (57.1) | 2 (28.6) | 1 (14.3) |
| Mucoepidermoid carcinoma | 4 | 1 (25.0) | 55 [49–57] | 3 (75.0) | 0 (0.0) | 1 (25.0) |
| Renal cell carcinoma | 3 | 3 (100) | 61 [59–73] | 0 (0.0) | 1 (33.3) | 2 (66.7) |
| Nonneoplastic salivary gland tissue | 6 | 2 (33.3) | 69 [62–86] | 6 (100) | 0 (0.0) | 0 (0.0) |
NR4A3 and DOG1 IHC results are summarized in Table 2 and in Figures 1 and 2. For NR4A3, 9 of 11 AcCC cases demonstrated nuclear positivity. Four cases (36.4%) showed 3+ intensity staining, involving 76% to 100% of cells in 2 cases and 51% to 75% of cells in 2 cases. Two cases (18.2%) showed 2+ intensity staining, involving 76% to 100% of cells in 1 case and 26% to 50% of cells in 1 case. Three cases (27.3%) showed 1+ intensity staining, involving 1% to 25% of cells in all 3 cases. Two cases (18.2%) showed no staining. Both AcCC specimens from the patient with high-grade transformation demonstrated NR4A3 IHC nuclear positivity, with 1+ staining in the primary tumor, and 3+ staining in the lymph node metastasis (for a representative images of lymph node metastasis, see Fig. 3). For the 2 negative AcCC cases, NR4A3 IHC on the corresponding surgical resection was positive in 1 case (indicating a false-negative on the cell block) and negative in 1 case (indicating a true-negative). All 19 non-AcCC neoplasms and all 6 instances of nonneoplastic SGT were NR4A3-negative by IHC. For the diagnosis of AcCC, NR4A3 IHC had 81.8% sensitivity and 100% specificity (see Table 3).
TABLE 2.
Nuclear Receptor Subfamily 4 Group A Member 3 and DOG1 Immunohistochemistry Results
| DOG1 IHC+: No. (%) | ||||
|---|---|---|---|---|
| Diagnosis | Case No. | NR4A3 IHC+: No. (%) | Any Staining | Complete Membranous Staining |
| Acinic cell carcinoma | 11 | 9 (81.8) | 9 (81.8) | 8 (72.7) |
| Neoplasm, not acinic cell carcinoma | 19 | 0 (0.0) | 7 (36.8) | 2 (10.5) |
| Oncocytoma | 3 | 0 (0.0) | 1 (33.3) | 0 (0.0) |
| Secretory carcinoma | 2 | 0 (0.0) | 1 (50.0) | 0 (0.0) |
| Salivary duct carcinoma | 7 | 0 (0.0) | 3 (42.9) | 2 (28.6) |
| Mucoepidermoid carcinoma | 4 | 0 (0.0) | 1 (25.0) | 0 (0.0) |
| Renal cell carcinoma | 3 | 0 (0.0) | 1 (33.3) | 0 (0.0) |
| Nonneoplastic salivary gland tissue | 6 | 0 (0.0) | 6 (100) | 0 (0.0) |
Abbreviation: IHC+, positive by immunohistochemistry; NR4A3, nuclear receptor subfamily 4 group A member 3.
Figure 1.
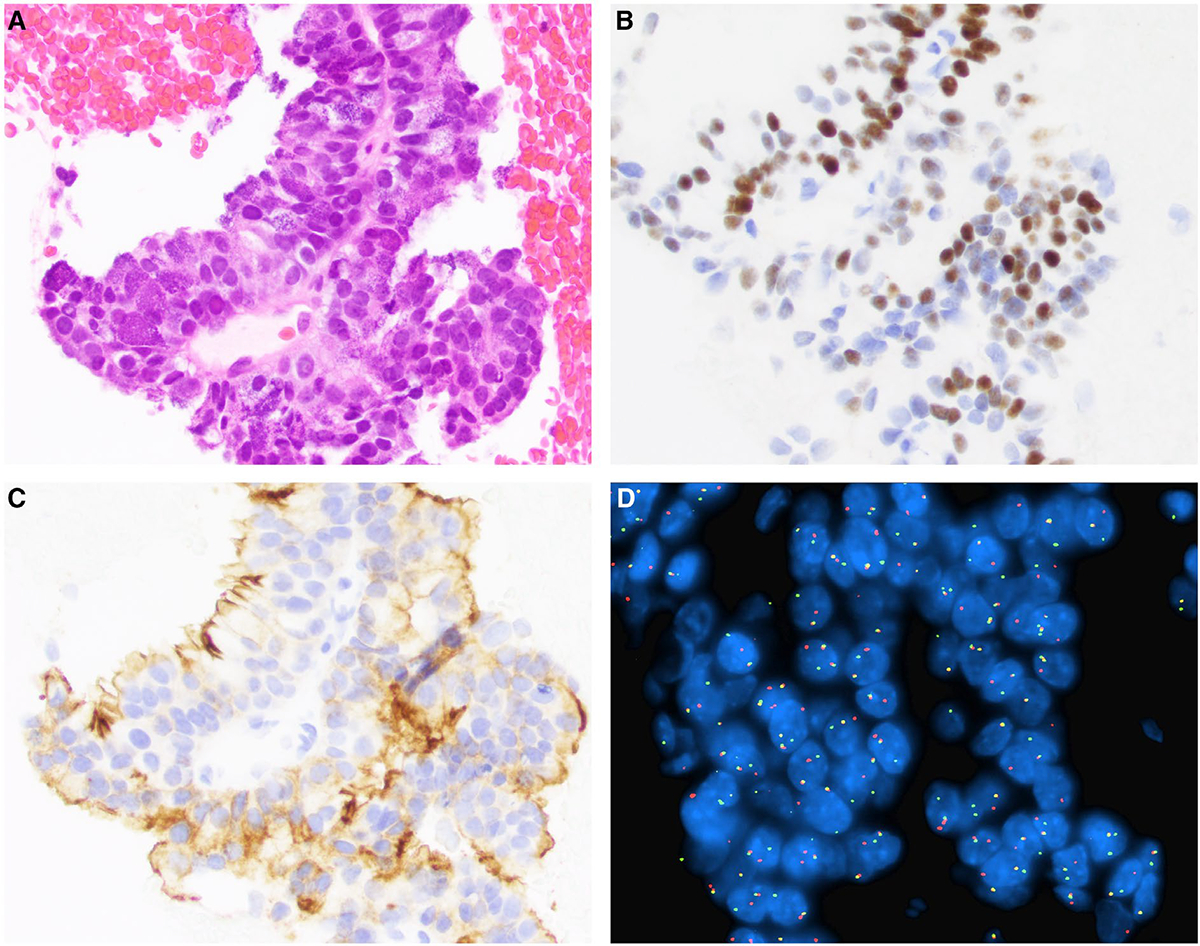
(A) Fine-needle aspiration of an acinic cell carcinoma is shown with (B) nuclear positivity for NR4A3 by immunohistochemistry, (C) apical membranous positivity for DOG1 by immunohistochemistry, and (D) nuclear receptor subfamily 4 group A member 3 (NR4A3) rearrangement by fluorescence in situ hybridization showing a break-apart signal (H&E stain; original magnification ×200 in A-C, ×400 in D).
Figure 2.
(A) Fine-needle aspiration of (top right) an acinic cell carcinoma (AcCC) (a different case than that shown in Fig. 1) has (bottom left) interspersed, nonneoplastic salivary gland epithelium. (B) AcCC cells are (top right) positive for NR4A3 by immunohistochemistry, whereas (bottom left) nonneoplastic salivary gland epithelial cells are negative (lower left). (C) AcCC cells show (top right) a combination of apical, complete membranous, and cytoplasmic staining for DOG1 by immunohistochemistry, whereas (bottom left) nonneoplastic salivary gland epithelial cells show a canalicular pattern of staining (H&E stain; original magnification ×200 in A-C).
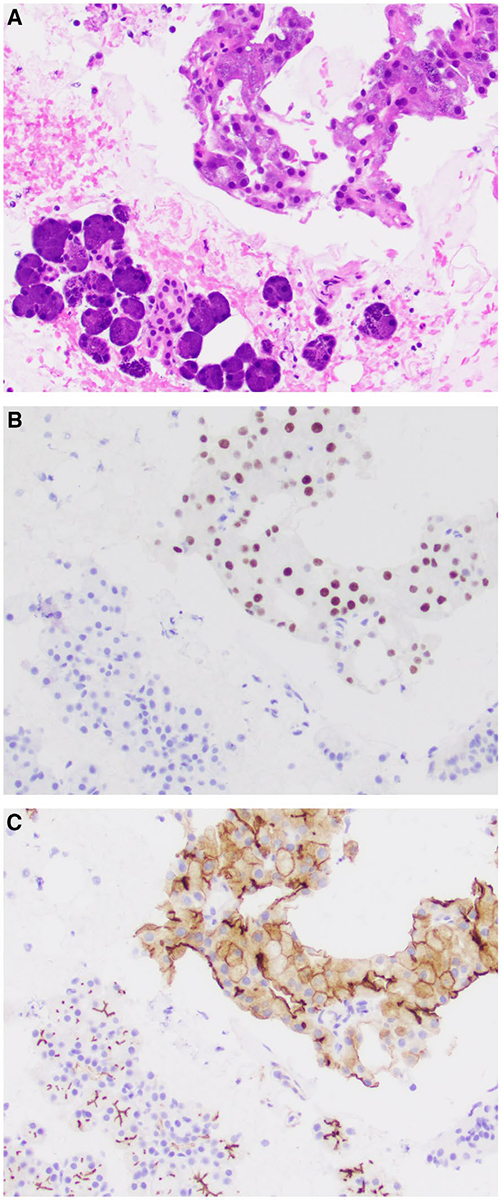
Figure 3.
(A) Fine-needle aspiration of a lymph node containing metastatic acinic cell carcinoma with high-grade transformation shows (B) nuclear positivity for nuclear receptor subfamily 4 group A member 3(NR4A3) by immunohistochemistry and (C) weak complete membranous positivity for DOG1 (H&E stain; original magnification ×400 in A-C).
TABLE 3.
Sensitivity and Specificity Data for NR4A3 Immunohistochemistry (IHC), DOG1 IHC, and Nuclear Receptor Subfamily 4 Group A Member Fluorescence In Situ Hybridization for the Diagnosis of Acinic Cell Carcinoma
| DOG1 IHC, % | ||||
|---|---|---|---|---|
| Variable | NR4A3 IHC, % | NR4A3 FISH, % | Any Staining | Complete Membranous Staining |
| Sensitivity | 81.8 | 36.4 | 81.8 | 72.7 |
| Specificity | 100.0 | 100.0 | 48.0 | 92.0 |
Abbreviations: FISH, fluorescence in-situ hybridization; NR4A3, nuclear receptor subfamily 4 group A member 3.
For DOG1 IHC, 9 (81.9%) AcCC cases demonstrated any positivity (at least 1+ staining of at least 1% of cells), and 8 of these 9 cases also demonstrated at least focal complete membranous staining. Of the 19 non-AcCC neoplastic cases (ie, excluding SGTs), 7 (36.8%) demonstrated any positivity, and 2 of these 7 also demonstrated at least focal complete membranous staining. All of the 6 SGT cases demonstrated the expected apical membranous staining pattern without any complete membranous staining. For the diagnosis of AcCC, DOG1 had a 81.8% sensitivity (any staining) or 72.7% sensitivity (complete membranous staining) and 48% specificity (any staining) or 92% specificity (complete membranous staining) (Table 3). For dual positivity with DOG1 and NR4A3 IHC, the sensitivity and specificity for the diagnosis of AcCC were 72.7% and 100%, respectively. For positivity with either stain, the sensitivity was 90.9% (any DOG1 staining) or 81.8% (complete membranous DOG1 staining), and the specificity was 48% (any DOG1 staining) or 92% (complete membranous DOG1 staining). In other words, the addition of DOG1 IHC did not improve the performance of NR4A3 IHC (Figs. 4, 5, and 6).
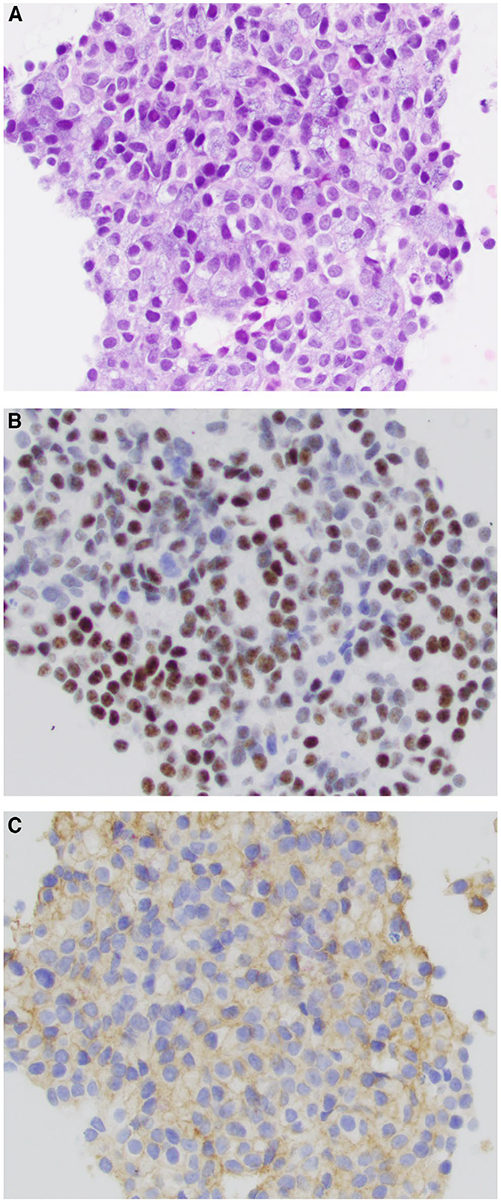
Figure 4.
(A) Fine-needle aspiration of oncocytoma is shown. By immunohistochemistry, oncocytoma cells are (B) negative for nuclear receptor subfamily 4 group A member 3(NR4A3) and (C) negative for DOG1 (H&E stain; original magnification ×200 in A-C).
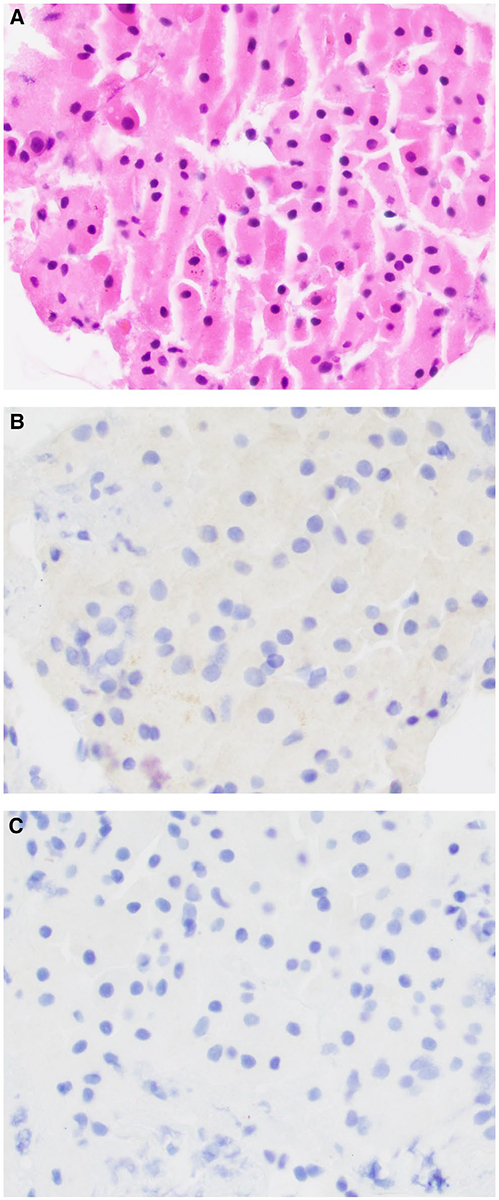
Figure 5.
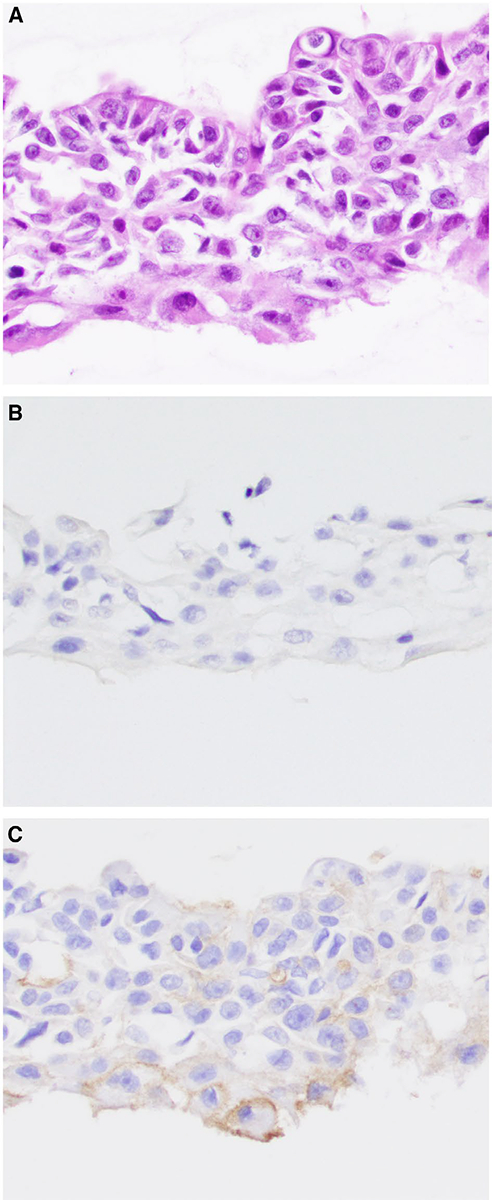
(A) Fine-needle aspiration of a salivary duct carcinoma is shown. By immunohistochemistry, the salivary duct carcinoma cells are (B) negative for nuclear receptor subfamily 4 group A member 3 (NR4A3) and (C) show patchy, membranous staining for DOG1 that is focally circumferential (bottom). The tumor cells were positive for androgen receptor by immunohistochemistry on the surgical resection (H&E stain; original magnification ×200 in A-C).
Figure 6.
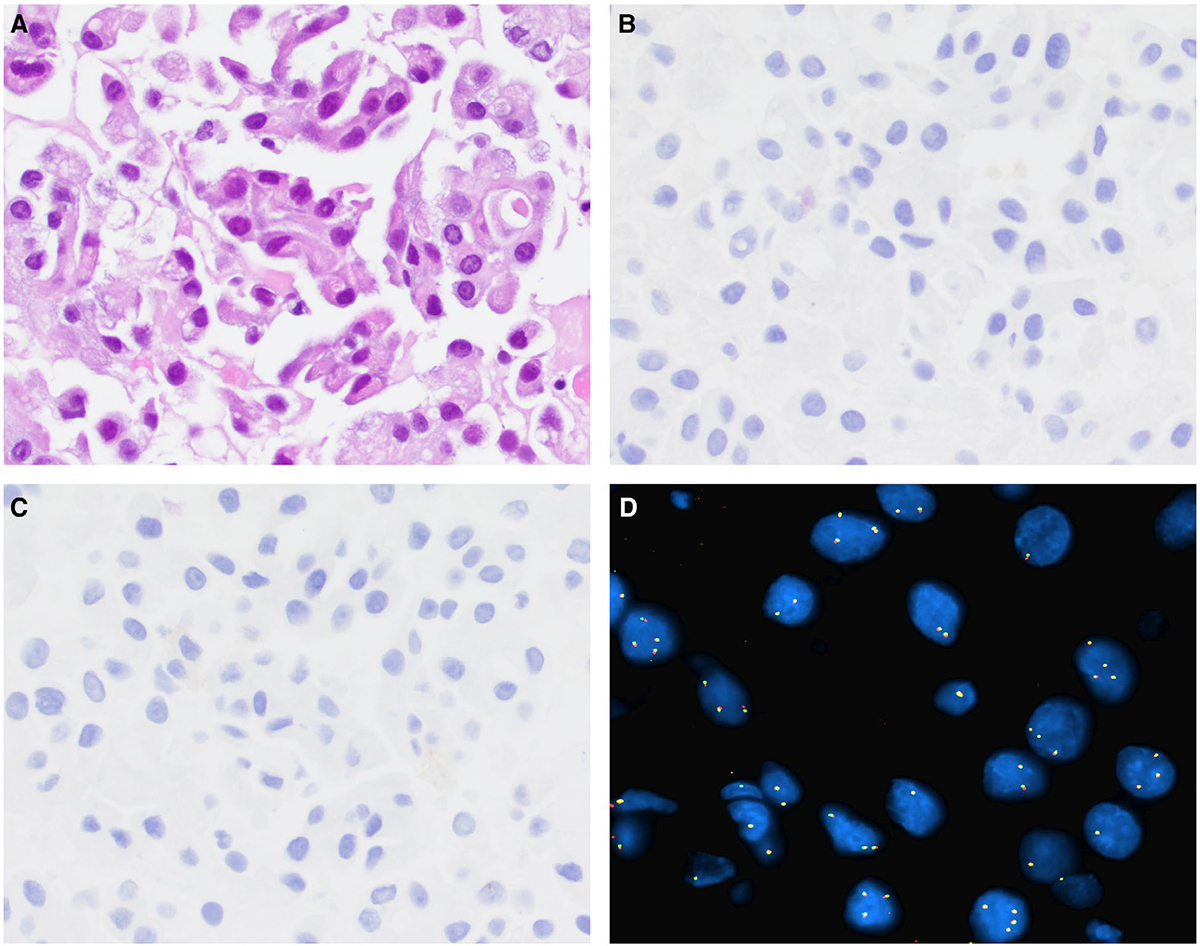
(A) Fine-needle aspiration of a secretory carcinoma is shown. By immunohistochemistry, secretory carcinoma cells are (B) negative for nuclear receptor subfamily 4 group A member 3 (NR4A3) and (C) negative for DOG1. (D) By fluorescence in situ hybridization, the tumor cells are negative for NR4A3 rearrangement, showing a lack of break-apart signal (H&E stain; original magnification ×200 in A-C, ×400 in D).
The NR4A3 FISH results are summarized in Table 4. Four AcCCs (36.4%) were positive for NR4A3 translocation using a 20% cutoff. All 4 were also positive for NR4A3 IHC, with at least 2+ intensity. Of the 7 NR4A3 FISH-negative cases, 5 were positive for NR4A3 IHC, including both the salivary gland and lymph node FNAs from the patient with high-grade transformation, whereas 2 were negative. Only 1 NR4A3 FISH-negative AcCC was MSANTD3 FISH-positive. A summary of all IHC and FISH results for the AcCC cytology cases and, if applicable, corresponding surgical resections, can be found in Table 5.
TABLE 4.
Nuclear Receptor Subfamily 4 Group A Member 3 Fluorescence In Situ Hybridization Results
| Diagnosis | Case No. | NR4A3 FISH+: No. (%) |
|---|---|---|
| Acinic cell carcinoma | 11 | 4 (36.4) |
| Neoplasm, not acinic cell carcinoma | 16 | 0 (0.0) |
| Oncocytoma | 3 | 0 (0.0) |
| Secretory carcinoma | 2 | 0 (0.0) |
| Salivary duct carcinoma | 7 | 0 (0.0) |
| Mucoepidermoid carcinoma | 1 | 0 (0.0) |
| Renal cell carcinoma | 3 | 0 (0.0) |
| Nonneoplastic salivary gland tissue | 5 | 0 (0.0) |
Abbreviation: FISH+, positive by fluorescence in-situ hybridization; NR4A3, nuclear receptor subfamily 4 group A member 3.
TABLE 5.
Summary of Immunohistochemistry and Fluorescence In-Situ Hybridization Results for All Acinic Cell Carcinoma Cytology Cases and Corresponding Surgical Resections, if Applicable
| Case No. | Cytology | Surgical | |||||
|---|---|---|---|---|---|---|---|
| DOG1 IHC (Any Staining) | DOG1 IHC (Complete Membranous) | NR4A3 IHC | NR4A3 FISH | NR4A3 IHC | NR4A3 FISH | MSANTD3 FISH | |
| 1 | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| 2 | Positive | Positive | Negative | Negative | Positive | Negative | Positive |
| 3a | Positive | Positive | Positive | Negative | Positive | Negative | Negative |
| 4 | Positive | Positive | Positive | Positive | Positive | Positive | Negative |
| 5 | Positive | Positive | Positive | Negative | Positive | Negative | Negative |
| 6 | Positive | Positive | Positive | Positive | NA | NA | NA |
| 7 | Negative | Negative | Positive | Negative | Positive | Negative | Negative |
| 8 | Positive | Positive | Positive | Positive | Positive | Positive | Negative |
| 9 | Positive | Positive | Positive | Negative | Positive | Negative | Negative |
| 10a | Positive | Positive | Positive | Negative | NA | NA | NA |
| 11 | Positive | Positive | Positive | Positive | NA | NA | NA |
Abbreviations: FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; MSANTD3, Myb/SANT-like DNA-binding domain containing 3; NA, not available; NR4A3, nuclear receptor subfamily 4 group A member 3.
Cases 3 (primary tumor) and 10 (lymph node metastasis) are from the same patient with high-grade transformation.
For 3 cases of MEC, NR4A3 FISH testing failed because of low cellularity. In 1 of the 2 cases of SGT admixed with neoplastic tissue (SDC), FISH analysis was not attempted on the SGT because of low cellularity on the section. All 21 successfully analyzed non-AcCC cases were negative for NR4A3 translocation. For the diagnosis of AcCC, NR4A3 FISH had 36.4% sensitivity and 100% specificity (Table 3).
DISCUSSION
The diagnosis of AcCC in cytology specimens can be difficult, particularly because of the morphologic overlap with several other entities and the suboptimal available ancillary studies. An accurate diagnosis is important because some entities in the differential diagnosis are nonneoplastic lesions or benign neoplasms, which potentially could be managed more conservatively. Although many salivary gland lesions are treated surgically, a definitive diagnosis of AcCC could clarify the treatment plan and allow for a more refined surgical plan (ie, potential inclusion of lymph node dissection). Historically, the primary IHC markers of AcCC have been DOG1 and SOX-10 (positive) and p63 or p40 (negative). However, this immunoprofile is not perfect, as discussed above, particularly with regard to cytology specimens in which critical histologic features may be lacking to aid in their interpretation. The identification of NR4A3 translocations and expression of NR4A3 by IHC allows for a more definitive diagnosis of AcCC. In addition to its higher sensitivity and specificity for AcCC, NR4A3 IHC positivity is nuclear, which is typically easier to interpret than IHC stains with membranous and cytoplasmic staining, such as DOG1. In addition, NR4A3 IHC has the advantage of being completely negative in SGTs, unlike DOG1 IHC.
The current study demonstrates the utility of NR4A3 IHC in cytology cell block preparations and its better performance than DOG1 IHC for the diagnosis of AcCC. Our results suggest that NR4A3 IHC may even be useful for cytologic diagnosis of AcCC with high-grade transformation. Also, it has been demonstrated that NR4A3 FISH is feasible on sufficiently cellular cytology cell block sections. The lack of myoepithelial neoplasms is a limitation of this study. Also, none of the AcCCs in this study were zymogen granule-poor The IHC findings are similar to those in a recently published study by Nguyen et al,38 who examined a similar number of AcCC cases. The current study, however, also includes a comparison with DOG1 IHC and NR4A3 FISH and an assessment of the corresponding resections with NR4A3 FISH and MSANTD3 FISH. Although the other study included more non-AcCC cases overall, most (n = 30) were PAs, an entity often readily distinguishable from AcCC by FNA. Also, the current study assesses more specimens in the cellular oncocytic/oncocytoid neoplasm category from the Milan system,3 such as SC, oncocytoma, SDC, and metastatic RCC.
The NR4A3 break-apart FISH probe used in this study and by Haller and colleagues34 does not identify all NR4A3 rearrangements. As Haller et al found in a subanalysis, 2 of 15 (13%) AcCCs with sequencing data showed a normal fusion signal by NR4A3 break-apart FISH. Both AcCCs with a normal NR4A3 fusion signal by FISH harbored the 9q31 breakpoint at genomic positions located in the middle of the green fluorescence probe, which explained why they showed intact fusion signals even in the presence of a 9q31 rearrangement. Interestingly, both were positive for NR4A3 IHC. The location of 9q31 breakpoints in the middle of the green fluorescence probe region, for some AcCCs, is 1 potential reason for the lower sensitivity of NR3A3 FISH than of NR4A3 IHC in our study. Recently, a case of AcCC without NR4A3 IHC expression or translocation reportedly demonstrated increased expression of NR4A2.39 This mechanism might explain why 1 AcCC case in the current series was negative for NR4A3 by IHC on both the cytology and surgical specimens.
Overall, we have shown that NR4A3 IHC and NR4A3 FISH are sensitive and specific markers in the diagnosis of AcCC and aid in differentiation from multiple cytologic mimickers of AcCC in cell block sections of cytology specimens. NR4A3 IHC is superior to NR4A3 FISH for the distinction of AcCC from its morphologic mimics on cytology.
FUNDING SUPPORT
This work was supported by departmental funds from the Department of Pathology at the University of Pittsburgh Medical Center.
We thank Kimberly Fuhrer, Megan Morrell, and Stacy Hicks for technical support, and Lynn Wolkenstein for administrative support.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, eds. WHO Classification of Head and Neck Tumours. WHO Classification of Tumours. 4th ed, Vol 9. International Agency for Research on Cancer; 2017. [Google Scholar]
- 2.Griffith CC, Pai RK, Schneider F, et al. Salivary gland tumor fine-needle aspiration cytology: a proposal for a risk stratification classification. Am J Clin Pathol. 2015;143:839–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faquin WC, Rossi ED, Baloch Z, et al. , eds. The Milan System for Reporting Salivary Gland Cytopathology. Springer; 2018. [Google Scholar]
- 4.Cibas ES, Ducatman BS, eds. Cytology: Diagnostic Principles and Clinical Correlates. 4th ed. Elsevier Saunders; 2014. [Google Scholar]
- 5.Jo VY, Krane JF. Ancillary testing in salivary gland cytology: a practical guide. Cancer Cytopathol. 2018;126(suppl 8):627–642. [DOI] [PubMed] [Google Scholar]
- 6.Evrard SM, Meilleroux J, Daniel G, et al. Use of fluorescent in-situ hybridisation in salivary gland cytology: a powerful diagnostic tool. Cytopathology. 2017;28:312–320. [DOI] [PubMed] [Google Scholar]
- 7.Ohtomo R, Mori T, Shibata S, et al. SOX10 is a novel marker of acinus and intercalated duct differentiation in salivary gland tumors: a clue to the histogenesis for tumor diagnosis. Mod Pathol. 2013;26:1041–1050. [DOI] [PubMed] [Google Scholar]
- 8.Hsieh MS, Lee YH, Chang YL. SOX10-positive salivary gland tumors: a growing list, including mammary analogue secretory carcinoma of the salivary gland, sialoblastoma, low-grade salivary duct carcinoma, basal cell adenoma/adenocarcinoma, and a subgroup of mucoepidermoid carcinoma. Hum Pathol. 2016;56:134–142. [DOI] [PubMed] [Google Scholar]
- 9.Lee JH, Kang HJ, Yoo CW, et al. PLAG1, SOX10, and Myb expression in benign and malignant salivary gland neoplasms. J Pathol Transl Med. 2019;53:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chenevert J, Duvvuri U, Chiosea S, et al. DOG1: a novel marker of salivary acinar and intercalated duct differentiation. Mod Pathol. 2012;25:919–929. [DOI] [PubMed] [Google Scholar]
- 11.Canberk S, Onenerk M, Sayman E, et al. Is DOG1 really useful in the diagnosis of salivary gland acinic cell carcinoma?—A DOG1 (clone K9) analysis in fine needle aspiration cell blocks and the review of the literature. Cytojournal. 2015;12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raboh NMA, Hakim SA. Diagnostic role of DOG1 and p63 immunohistochemistry in salivary gland carcinomas. Int J Clin Exp Pathol. 2015;8:9214–9222. [PMC free article] [PubMed] [Google Scholar]
- 13.Khurram SA, Speight PM. Characterisation of DOG-1 expression in salivary gland tumours and comparison with myoepithelial markers. Head Neck Pathol. 2019;13:140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu S, Schuerch C, Hunt J. Review and updates of immunohistochemistry in selected salivary gland and head and neck tumors. Arch Pathol Lab Med. 2015;139:55–66. [DOI] [PubMed] [Google Scholar]
- 15.Bundele MM, Weinreb I, Xu B, et al. Mucoacinar carcinoma: a rare intercalated duct/acinar variant of mucoepidermoid carcinoma, hybrid tumor, or distinct entity? Mod Pathol. 2016;29:322A. [Google Scholar]
- 16.Skalova A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of the salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34:599–608. [DOI] [PubMed] [Google Scholar]
- 17.Fehr A, Loning T, Stenman G. Mammary analogue secretory carcinoma of the salivary glands with ETV6-NTRK3 gene fusion. Am J Surg Pathol. 2011;35:1600–1602. [DOI] [PubMed] [Google Scholar]
- 18.Tonon G, Modi S, Wu L, et al. T(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat Genet. 2003;33:208–213. [DOI] [PubMed] [Google Scholar]
- 19.Fehr A, Roser K, Heidorn K, Hallas C, Loning T, Bullerdiek J. A new type of MAML2 fusion in mucoepidermoid carcinoma. Genes Chromosomes Cancer. 2008;47:203–206. [DOI] [PubMed] [Google Scholar]
- 20.Persson M, Andren Y, Mark J, Horlings HM, Persson F, Stenman G. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci U S A. 2009;106:18740–18744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brayer KJ, Frerich CA, Kang H, Ness SA. Recurrent fusions in MYB and MYBL1 define a common, transcription factor-driven oncogenic pathway in salivary gland adenoid cystic carcinoma. Cancer Discov. 2016;6:176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonescu CR, Katabi N, Zhang L, et al. EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosomes Cancer. 2011;50:559–570. [DOI] [PubMed] [Google Scholar]
- 23.Kas K, Voz ML, Roijer E, et al. Promoter swapping between the genes for a novel zinc finger protein and B-catenin in pleiomorphic adenomas with t(3;8)(p21;q12) translocations. Nat Genet. 1997;15:170–174. [DOI] [PubMed] [Google Scholar]
- 24.Geurts JM, Schoenmakers EF, Roijer E, Stenman G, Van de Ven WJM. Expression of reciprocal hybrid transcripts of HMGIC and FHIT in a pleomorphic adenoma of the parotid gland. Cancer Res. 1997;57:13–17. [PubMed] [Google Scholar]
- 25.Geurts JM, Schoenmakers EF, Roijer E, Astrom AK, Stenman G, Van de Ven WJM. Identification of NFIB as recurrent translocation partner gene of HMGIC in pleomorphic adenomas. Oncogene. 1998;16:865–872. [DOI] [PubMed] [Google Scholar]
- 26.Martins C, Fonseca I, Roque L, et al. PLAG1 gene alterations in salivary gland pleomorphic adenoma and carcinoma ex-pleomorphic adenoma: a combined study using chromosome banding, in situ hybridization and immunocytochemistry. Mod Pathol. 2005;18:1048–1055. [DOI] [PubMed] [Google Scholar]
- 27.Kandasamy J, Smith A, Diaz S, Rose B, O’Brien C. Heterogeneity of PLAG1 gene rearrangements in pleomorphic adenoma. Cancer Genet Cytogenet. 2007;177:1–5. [DOI] [PubMed] [Google Scholar]
- 28.Bahrami A, Perez-Ordonez B, Dalton JD, Weinreb I. An analysis of PLAG1 and HMGA2 rearrangements in salivary duct carcinoma and examination of the role of precursor lesions. Histopathology. 2013;63:250–262. [DOI] [PubMed] [Google Scholar]
- 29.Katabi N, Ghossein R, Ho A, et al. Consistent PLAG1 and HMGA2 abnormalities distinguish carcinoma ex-pleomorphic adenoma from its de novo counterparts. Hum Pathol. 2015;46:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiosea SI, Thompson LD, Weinreb I, et al. Subsets of salivary duct carcinoma defined by morphologic evidence of pleomorphic adenoma, PLAG1 or HMGA2 rearrangements, and common genetic alterations. Cancer. 2016;122:3136–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Hallani S, Udager AM, Bell D, et al. Epithelial-myoepithelial carcinoma: frequent morphologic and molecular evidence of preexisting pleomorphic adenoma, common HRAS mutations in PLAG1-intact and HMGA2-intact cases, and occasional TP53, FBXW7, and SMARCB1 alterations in high-grade cases. Am J Surg Pathol. 2018;42:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barasch N, Gong X, Kwei KA, et al. Recurrent rearrangements of the Myb/SANT-like DNA-binding domain containing 3 gene (MSANTD3) in salivary gland acinic cell carcinoma. PLoS One. 2017;12:e0171265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haller F, Bieg M, Will R, et al. Enhancer hijacking activates oncogenic transcription factor NR4A3 in acinic cell carcinomas of the salivary glands. Nat Commun. 2019;10:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haller F, Skalova A, Ihrler S, et al. Nuclear NR4A3 immunostaining is a specific and sensitive novel marker for acinic cell carcinoma of the salivary glands. Am J Surg Pathol. 2019;43:1264–1272. [DOI] [PubMed] [Google Scholar]
- 35.Baratelli F, Balzer B, Wonwoo S. Nuclear NR4A3 expression distinguishes low-grade acinic cell carcinoma from anorexia/bulimia-related sialadenosis of the salivary gland. Paper presented at: United States and Canadian Academy of Pathology (USCAP) 109th Annual Meeting; February 29 to March 5; USCAP 2020. Los Angeles, CA: Mod Pathol, 2020:1183. [Google Scholar]
- 36.Wong K, Jo V, Hornick J. NR4A3 immunohistochemistry reliably discriminates acinic cell carcinoma from its mimics. United States & Canadian Academy of Pathology 109th Annual Meeting Abstracts. Mod Pathol. 2020;33(suppl):1239. [Google Scholar]
- 37.Andreasen S, Varma S, Barasch N, et al. The HTN3-MSANTD3 fusion gene defines a subset of acinic cell carcinoma of the salivary gland. Am J Surg Pathol. 2019;43:489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen L, Chopra S, Laskar DB, et al. NOR-1 distinguishes acinic cell carcinoma from its mimics on fine needle aspiration biopsy specimens. Hum Pathol. 2020;102:1–6. [DOI] [PubMed] [Google Scholar]
- 39.Haller F, Moskalev EA, Kuck S, et al. Nuclear NR4A2 (Nurr1) immunostaining is a novel marker for acinic cell carcinoma of the salivary glands lacking the classic NR4A3 (NOR-1) upregulation. Am J Surg Pathol. Published online April 24, 2020. doi: 10.1097/PAS.0000000000001494 [DOI] [PubMed] [Google Scholar]


