Fig. 4. Conversion of pseudocycle binders into ligand-gated channels and CID systems.
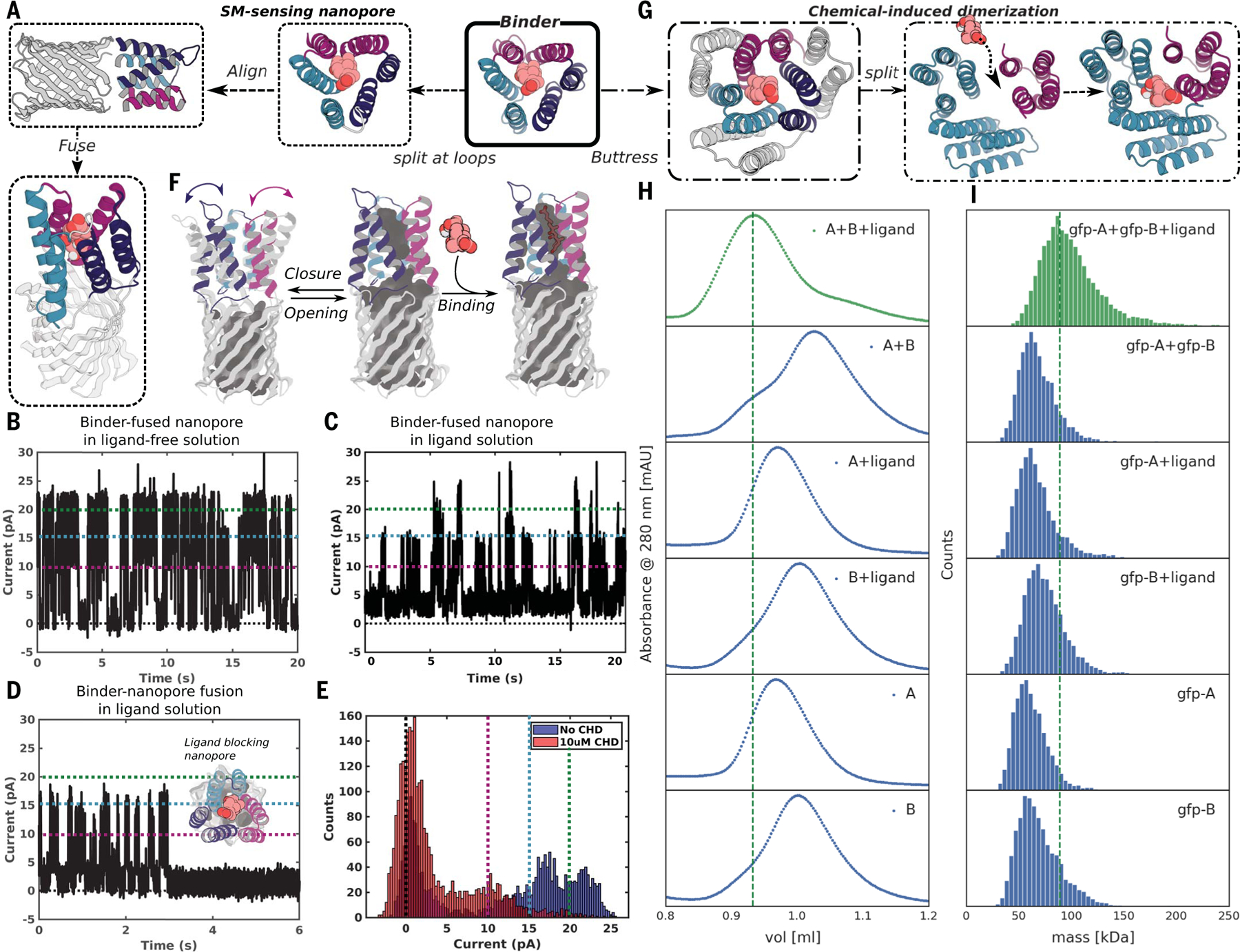
(A to F) Ligand-sensing de novo nanopore construction. (A) shows how the three structural repeat units of CHD_r1 were inserted into three different loops in a 12-stranded de novo nanopore by using inpainting to join the chains, such that the central axes of binder and nanopore are aligned. The conductance of the original nanopore is ~220 pS (fig. S17A) and is not influenced by CHD. The conductance of binder-fused nanopore in the absence (B) and presence of CHD [(C) to (E)] are shown for comparison. In the absence of CHD (B), the pore fluctuates between a state with high conductance very similar to the unmodified pore and a low-conductance state (C); in the presence of CHD, the duration of the low-conductance states is greatly increased [(C) to (E)]; the longer record (C) and a single closure event (D) are shown for clarity, and the histogram of the current with and without ligand is shown in (E). Different currents (0, 10, 15, and 20 pA) are marked out for clarity using dashed lines in black, magenta, teal, and green, respectively in (B) to (E). The gated nanopores are robust through multiple cycles of opening and closure (C). Upon reversal of the voltage, the original high-conductance state is restored (fig. S19B). As indicated schematically in (F), the conductance fluctuations of the binder-fused pore in absence of ligand likely reflect transient association of the three subunits; ligand binding stabilizes the associated state leading to prolonged blocking of the pore. (G to I) CID system construction. The CHD binder, CHD_r1, was buttressed by diffusion of an outer ring of helices to increase the stability of split protein fragments (Fig. 2, C to F, and figs. S7B and S20). To create a CID system, we split the buttressed binders into halves and redesign the protein-protein interface to increase solubility of the fragments and disfavor association in the absence of ligand. Characterization of CHD induced association of the split fragments by SEC (H) and mass photometry (I). Dimerization of the two split domains (A and B) in presence (first trace from top), but not the absence, (second trace from top) of ligand. The individual monomers do not dimerize in the presence (third and fourth trace from top) or absence (fifth and sixth traces from the top) of ligands. N-terminal GFP tags were fused to the monomers to facilitate detection by mass photometry.
