Abstract
Over the past 15 years, sex-related differences in aortic valve (AV) stenosis (AS) have been highlighted, affecting various aspects of AS, such as the pathophysiology, AV lesions, left ventricle remodelling, and outcomes. Female patients were found to present a more profibrotic pattern of leaflet remodelling and/or thickening, whereas male patients have a preponderance of calcification within stenosed leaflets. The understanding of these sex differences is still limited, owing to the underrepresentation of female patients in many basic and clinical research studies and trials. A better understanding of sex differences in the pathophysiology of AS may highlight new therapeutic targets that potentially could be sex-specific. This review aims to summarize sex-related differences in AS, as discovered from basic research experiments, covering aspects of the disease ranging from leaflet composition to signalling pathways, sex hormones, genetics and/or transcriptomics, and potential sex-adapted medical treatments.
Graphical abstract

Résumé
Au cours des 15 dernières années, des différences liées au sexe dans la sténose (SA) de la valve aortique (VA) ont été mises en évidence, affectant divers aspects de la SA, tels que la pathophysiologie, les lésions de la VA, le remodelage du ventricule gauche et les pronostics associés. Il a été constaté que les patientes présentaient un patron plus profibrotique du remodelage des feuillets ou de leur épaississement, tandis que les patients de sexe masculin voyaient une prépondérance de la calcification au sein des feuillets sténosés. La compréhension de ces différences entre les sexes est encore limitée, en raison de la sous-représentation des femmes dans de nombreuses études et essais de recherche fondamentale et clinique. Une meilleure compréhension des différences entre les sexes dans la physiopathologie de la SA pourrait mettre en évidence de nouvelles cibles thérapeutiques qui pourraient être spécifiques au sexe. Cette revue vise à résumer les différences liées au sexe dans la SA, telles qu'elles ont été découvertes par des expériences de recherche fondamentale, couvrant des aspects de la maladie allant de la composition du feuillet aux voies de signalisation, aux hormones sexuelles, à la génétique ou à la transcriptomique, ainsi qu'aux traitements médicaux potentiels adaptés en fonction du sexe.
Calcified aortic valve (AV) stenosis (AS) is the most common valvular heart disease in high-income countries. AS occurs in about 5% of the population aged > 65 years, and in 10% of people aged > 80 years.1,2 In 2017, 12.6 million people were diagnosed with AS,3 and the number of cases has increased over the years.4 Patients diagnosed with severe AS are at high risk of heart failure and/or death within 5 years from diagnosis,5 but no medical therapy is available. The only option is to replace the stenotic AV through surgical or transcatheter approaches.6, 7, 8, 9 Each year, more than 200,000 AV replacements and 20,000 deaths associated with AS are recorded in North America.4,10 Thus, AS carries a significant economic and societal burden, and the need to develop alternative treatments to alleviate these burdens is urgent.
Many sex differences have been highlighted in AS, from clinical studies. AVs in female patients have more fibrosis and less calcification than do those in male patients, for the same hemodynamic parameters and the same severity of AS.11,12 Differences also occur in the remodelling of the left ventricle, with more concentric hypertrophy in female patients, and more eccentric hypertrophy in male patients,13 which leads to a sex-specific presentation of more low-flow with preserved ejection fraction in female patients.14 These discoveries have been explained at the molecular level, but many mechanisms remain unexplained (Fig. 1). Several hypotheses have been proposed regarding major sex-specific factors in the development of AS, including lipid metabolism, immunity, hormones, and genetics. In this article, we aim to review the molecular mechanisms associated with AS, and we then focus on molecular differences between male and females, as highlighted in studies conducted mainly in experimental models.
Figure 1.
Sex-related differences observed in aortic stenosis (AS). VECs, valvular endothelial cells; VICs, valvular interstitial cells. Figure created with BioRender.com.
Composition of the AV
Human AV leaflets are trilayered structures, each assuring different properties. The fibrosa, facing the aorta, is mainly composed of collagen fibers oriented circumferentially, which provides tensile stiffness.15 The ventricularis, facing the left ventricle, is a layer of elastic fibers that gives the leaflets their compliance. Finally, the spongiosa, which separates the fibrosa from the ventricularis, is rich in proteoglycans and glycosaminoglycans, giving the leaflets elasticity and flexibility.16 The AV is mainly composed of valvular endothelial cells (VECs), valvular interstitial cells (VICs), immune cells, and extracellular matrix (ECM), all of which play important roles in physiological and pathologic conditions (Fig. 2). All of these components may undergo and regulate calcification and fibrosis processes in a sex-specific manner.
Figure 2.
Pathophysiology of aortic stenosis (AS) and possible impact of sex hormones and sex differences. An endothelial lesion induces the infiltration of lipids, quickly followed by their oxidation, and inflammatory cells in the fibrosa. The renin-angiotensin-aldosterone system (RAAS) regulates valvular inflammation and seems to be anti-inflammatory in female patients through the effect of estrogen, and proinflammatory in male patients via testosterone. The transforming growth factor-β (TGF-β) pathway, upregulated in female patients, may promote the endothelial-mesenchymal transition (EndoMT) observed in AS. Valvular interstitial cells (VICs) are activated by several factors, including oxidized lipids. Their osteogenic transition seems to be inhibited by estrogen and promoted by testosterone, resulting in more calcification in stenotic aortic valves in male patients than in female patients. Moreover, the Ak strain transforming (Akt) pathway inhibits calcification in female patients, whereas the nuclear factor kappa beta (NFκB) pathway favours it through the receptor activator of nuclear factor κ B, receptor activator of nuclear factor kappa-B ligand, osteoprotegerin (RANK/RANKL/OPG) pathway in male patients. At the same time, chymase, produced by mast cells, and angiotensin-converting enzyme (ACE) induce fibrosis through the RAAS. Deposition of fibrosis is favoured by TGF-β1/β2 and wingless-related integration site (Wnt), a pathway upregulated by estrogen and potentially by testosterone. Vascular endothelial growth factor (VEGF) is an angiogenic factor secreted by male and female VICs in AS. In female patients, basic fibroblast growth factor (bFGF) also seems to promote angiogenesis. Gla, Matrix Gla protein ; ox, oxidized; PL, phospholipids; ROS, reactive oxygen species; VEC, valvular endothelial cells; VEGF, vascular endothelial growth factor; oVIC, osteoblastic VICs ; qVIC, quiescent VICs. Figure created with BioRender.com.
Valvular endothelial cells
VECs form a protective monolayer17 over the 2 faces of the AV.15 In physiological conditions, these cells have multiple functions, such as secreting nitric oxide to regulate vascular homeostasis,17,18 and replenishing the population of VICs.17,19 VECs located on the ventricularis face experience shear stress from blood flow, whereas those on the fibrosa face are exposed to oscillatory shear stress. Under pathologic conditions, such as oscillatory shear stress, and inflammation, VECs may differentiate into osteoblasts via an endothelial–mesenchymal transition.20,21 VECs also may inhibit the calcification of VICs by promoting the anti-osteogenic effect of NOTCH1.22
Sex differences in VECs are generally understudied. A recent study, on normal porcine AVs, observed that the proliferation of VECs differs between male and female individuals. Healthy male VECs exhibit a higher level of proliferation than do female VECs in vitro.23 This difference might be due to higher levels of thrombospondin 2 being secreted by female VECs.23
Valvular interstitial cells
VICs are located throughout the AV leaflets, beneath the VEC layer. VICs have 4 subpopulations, as follows: progenitor; quiescent; activated; and osteogenic.24 Progenitor VICs may originate from endothelial and hematopoietic lineages.24, 25, 26, 27 Progenitors from an endothelial origin may give rise to quiescent VICs, whereas those from a hematopoietic origin may give rise to activated VICs involved in valve repair.24 Quiescent VICs (qVICs) maintain the balance between the synthesis and degradation of ECM components in the valve under physiological conditions.21 In response to physiological stimuli, qVICs may get activated into myofibroblast cells, ensuring normal ECM remodelling and function.21 This differentiation is thought to be reversible when stimuli recede.21,28 Under pathologic conditions, qVICs and progenitor VICs (from both endothelial and hematopoietic origins) may transform into activated VICs (aVICs) capable of repairing the valve.24 They may evolve into preosteoblasts or myofibroblasts promoting calcification.25 This transition is associated with ECM remodelling and contributes to the fibro-calcific remodelling of the AV.29 VICs might lose their pluripotency when AV calcification develops. In vitro experiments show that VICs extracted from normal valves can differentiate into myofibroblastic, osteoblastic, chondrogenic, or adipogenic lineages,30 whereas VICs from calcified valves are less prone to differentiation into other cell types.31
Other studies provide complementary data that help in understanding the molecular mechanisms associated with sex differences in VICs. Male porcine VICs grown in osteogenic medium form bigger and more-dense nodules of calcium than do female VICs.32 Also, male, compared to female, rat VICs express increased early osteogenic markers.32 Female porcine VICs may have greater metabolic activity and collagen production than do male VICs,33 but these later data seem controversial.32 Altogether, these data suggest that stronger and earlier calcifying events occur in male VICs, and that more fibrotic events occur in female VICs. Thus, VICs may be important protagonists in sex-specific calcification and/or fibrosis processes.
An important finding is that VICs also may play a role in angiogenesis in a sex-specific manner. Female porcine VICs show a higher secretion level of vascular endothelial growth factor A (VEGF-A) than do male VICs, but only in qVICs. Basic fibroblast growth factor (also called FGF-2) production also tends to be higher in female porcine VICs.23 However, VEGF-A is increased in AVs from male patients with severe AS, compared to that in female patients (Fig. 2).34 Female porcine aVICs have more heparan sulfate proteoglycan-2,23 a molecule that favours growth factor binding. All these studies show discrepancies between male and female VICs in angiogenesis, but further investigations are needed to clarify the conflicting results.
Other cell populations in the AV
A recent transcriptomic study differentiated 14 cell subtypes in stenotic AVs. Beside VIC and VEC subpopulations, 6 valve-derived stromal cell populations and 3 immune-derived cell populations were identified.20 Macrophages, mast cells, and lymphocytes have been found to promote inflammation in stenotic AVs.35,36 Smooth muscle cells represent a small proportion of AV cells and are found mostly in the ventricularis layer. In AS, smooth muscle cells are found near the calcified areas.37
Sex differences also may be present in these cell populations. A study from Myasoedova et al. recently showed that stenosed AVs from female individuals contain more mesenchymal cell signatures, whereas those from male individuals have more signatures from macrophages, monocytes, T cells, and B cells.38 Another study conducted on human VICs shows that the osteogenic response induced by interferon-α and/or lipopolysaccharide is counteracted by the Ak strain transforming (Akt) pathway in female but not male VICs.39 This result indicates that not only valve composition but also immune response may be sex specific in AS.
Extracellular matrix
The ECM differs in the 3 layers of aortic leaflets, as follows: the fibrosa is rich in collagen I and III; the spongiosa is rich in glycosaminoglycans; and the ventricularis is rich in elastin.40 All these molecules confer specific mechanical properties to the AV.41 In pathologic conditions, collagen fibers become degraded and disorganized, and they interact with calcified vesicles.41 Several matrix metalloproteinases (MMPs), such as MMP1, MMP7, MMP9, and MMP12, are upregulated, whereas tissue inhibitor of metalloproteinase (TIMP) 4 is downregulated.42
The composition of the ECM is important in AS because its stiffness modifies the proliferation, differentiation, and viability of porcine VICs cultured in osteogenic media.43 An interesting finding is that in cell culture, ECM composition is modulated by the sex of the VICs. In male individuals, the levels of glycosaminoglycans, collagen I, and activated MMP2 are higher than those in female individuals.32 In female individuals, a higher level of expression and production of MMP2, MMP3, and MMP9, and a lower activity level of collagenase and gelatinase are observed, which is concordant with an accumulation of ECM.33 It is important to note that 17β-estradiol inhibits the transcription of MMP2 through the mitogen-activated protein kinase extracellular signal-regulated kinase 1/2 (MAPK-ERK1/2) signalling pathway in cardiac fibroblasts, suggesting that estrogens play a role in ECM remodelling.44
Pathophysiology of Calcified AS
The causes of AS are mainly unknown, but several mechanisms involved in the pathophysiology of the disease, including shear stress due to pressure overload, inflammation, and oxidative stress, have been elucidated.45 AS initiation appears when an endothelial lesion is observed.46 This initiation is followed by lipid infiltration, mainly of low-density lipoproteins and lipoprotein (a), which triggers the expression of Toll-like receptors on VICs.46 Reactive oxygen species are produced, oxidizing lipoproteins, which in turn induce the apoptosis of VICs and a cascade of immune reactions.46 Many factors, such as the expression of cell adhesion molecule,46 the recruitment of macrophages, CD11b,47 and major histocompatibility complex (MHC) class II dendritic cells, are observed.48 CD4+ and CD8+ T lymphocytes are attracted, mostly near areas of calcification or neovessels.49 Mast cells secrete chymase, an enzyme promoting the production of angiotensin II and fibrosis in the AV.50 Other mediators of inflammation, such as interleukin (IL)-6, cyclooxygenase 2, intracellular adhesion molecule 1 (ICAM1), and transforming growth factor beta-1 (TGF-β1), participate in the pathophysiology of AS (Fig. 2).25,46,51
Lipid infiltration, oxidative stress, and inflammation favour the differentiation of VICs, VECs, and macrophages into aVICs, a myofibroblast-like subpopulation of cells.52 Myofibroblasts produce collagen,53 a major constituent of fibrosis, and aggregate in apoptotic nodules; calcium deposits are produced around collagen fibers.25 Concomitantly, VICs differentiate into osteoblasts responsible for the formation of true bone inclusions25 and express markers of osteoblastic differentiation, such as Runt-related transcription factor 2 (RUNX2), bone morphogenic protein (BMP), alkaline phosphatase (ALP), and osteocalcin.25,46 For unknown reasons, the pathophysiology of the disease shows a significant imbalance between male and female patients.12 Male patients develop more calcifications, whereas female patients develop more fibrosis, for comparable hemodynamic parameters,12 regardless of the phenotype of the AV.54 An interesting point to note is that the immune response system may influence the fibrotic and calcific phenotypes observed, because immune cells such as monocytes, macrophages, T cells, and B cells are enriched in male individuals, whereas mesenchymal cells are enriched in female individuals.38
Signalling pathways
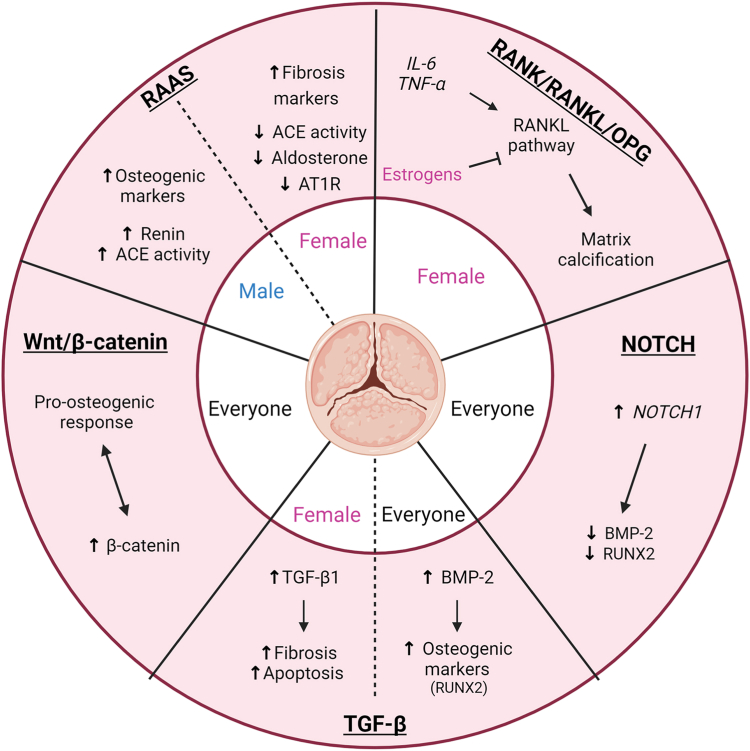
Various pathways are involved in AS. The renin-angiotensin-aldosterone system (RAAS) regulates blood pressure, and its chronic activation induces systemic hypertension, which has been linked to calcification in AS.55 The receptor activator of nuclear factor κ B, receptor activator of nuclear factor kappa-B ligand, osteoprotegerin (RANK/RANKL/OPG) signalling pathway promotes the inflammatory response and calcification observed in AS. The Notch signalling pathway inhibits calcification, whereas the transforming growth factor-β (TGF-β) pathway favours the accumulation of fibrosis. The role of the wingless-related integration site (Wnt)/β-catenin pathway in AS needs deeper research, but it seems to regulate both fibrosis and calcification. These 5 pathways contribute to the development of AS and may be sex-specific (Fig. 3).
Figure 3.
Sex differences in pathways involved in aortic stenosis (AS), and possible impact of sex hormones. Pathways leading to calcification and fibrosis may be modulated by sex hormones. In female patients, fibrosis markers may be increased through the renin-angiotensin-aldosterone system (RAAS), whereas in male patients, calcification markers may be upregulated. Estrogens may play a protective role in the calcification of the aortic valve by inhibiting the receptor activator of nuclear factor κ B (RANK) pathway. The Notch pathway may favour calcification processes and may be regulated by sex hormones, but no evidence has been found regarding its sex-specific regulation in AS. Several pieces of evidence show that transforming growth factor-β (TGF-β) may favour fibrosis in female patients. The wingless-related integration site (Wnt/β)-catenin pathway may favour a pro-osteogenic response in AS, but the involvement of sex hormones in its regulation needs to be clarified. ACE, angiotensin-converting enzyme; AT1R, angiotensin II type I receptor; BMP-2, bone morphogenic protein 2; IL-6, interleukine-6; OPG, osteoprotegerin; RANKL, receptor activator of nuclear factor kappa-B ligand; RUNX2, runt-related transcription factor 2; TNF-α, tumour necrosis factor alpha. Figure created with BioRender.com.
RAAS
Systolic hypertension is often concomitant with AS and is associated with faster progression of AV calcification.56 Aldosterone and angiotensin are 2 molecules in this pathway that may be involved in AS in a sex-specific manner. In male VICs, aldosterone increases the expression of calcification markers, such as BMP2, BMP4, periostin, and osteopontin, through the mineralocorticoid receptor. However, in female VICs, aldosterone increases fibrosis markers (fibronectin, lumican, tissue inhibitor of metalloproteinase [TIMP]-1) via the same receptor.57
Under physiological conditions, angiotensin II regulates several parameters through its receptors AT1 and AT2.58 Through AT1, it promotes vasoconstriction, fibrosis, inflammation, and oxidative stress, whereas through AT2, it promotes the opposite.58 An interesting finding is that the AT1-receptor is the only receptor in the AV. Angiotensin II is produced in the AV via 2 enzymes—chymase and angiotensin-converting enzyme (ACE), both of which are overexpressed in stenotic AVs.50,59 A recent study also showed that, in mice, treatment with angiotensin II increases the amount of collagen fibers (trend only).60 The same work revealed that angiotensin II increases proliferation, activation, fibrosis, and TGF-β1 expression in VICs, but no information is given about the sex of the mice or the cells. Another study in mesangial cells suggests that angiotensin II may have a profibrotic effect by increasing the expression of ECM proteins through the induction of TGF-β.61 At the physiological level, patients with AS are known to present high plasma levels of angiotensin II,55 which is associated with high expression of inflammatory markers (IL-6 and tumour necrosis factor-α) in the AV.62 These data suggest that RAAS may be involved in promoting valvular inflammation and fibrosis.
Knowledge is lacking regarding the sex-specific action of angiotensin II within the AV at the cellular level. Nevertheless, some data show that estrogen and testosterone may modulate the RAAS and thus modulate AS pathophysiology (Fig. 2). Estrogens promote high angiotensinogen levels (the precursor of angiotensin), low renin levels, ACE activity, aldosterone production, and angiotensin II type 1 receptor (AT1R) expression.63 They downregulate the angiotensin I receptor in vascular smooth muscle cells.64 In premenopausal female individuals, the RAAS seems to be cardioprotective via interactions with estrogens,65,66 but intriguingly, an increase in the expression of angiotensinogen is observed in women treated with either estrogen-replacement therapy or contraceptive therapy.67 Moreover, some studies show that RAAS may be influenced by hormonal changes during the menstrual cycle and in postmenopausal women.68 In postmenopausal women, estrogen deficiency also deregulates the RAAS and leads to a proinflammatory state.69
In contrast, testosterone tends to increase renin levels and ACE activity.70 In male individuals, testosterone can stimulate both vasodilator and vasoconstrictor pathways.58,71 In contrast to estrogen, testosterone seems to amplify the pathologic features of the RAAS in cardiovascular diseases by favouring the angiotensin II-ACE-AT1 receptor pathway.58 Estrogen and testosterone could partially explain the sex-related differences in the RAAS, but their effects seem to be tissue-specific and have not yet been studied in the AV (Fig. 3).
RANK/RANKL/OPG
This pathway is a well known major contributor to bone homeostasis. As calcification is an important feature of AS, this pathway is of great interest. Studies in VICs show that the RANK/RANKL/OPG pathway is a downstream effector of tumour necrosis factor-α and IL-6, which promote calcification.72, 73, 74 RANKL also promotes matrix calcification in human VICs and their osteogenic differentiation.75 In vascular smooth muscle cells, RANKL is induced by oxidative stress and is under the control of RUNX2, a well known marker of calcification.76
RANKL is enhanced in the stenosed AVs of male, compared to female, individuals, suggesting that it has a role in the calcification of the AV in male individuals.77 Even though female stenosed AVs have more fibrosis, they still exhibit some calcifications,12,78 so the study of calcification in female AS is still of interest. In human aortic endothelial cells, and human aortic smooth muscle cells, estrogen interacts with estrogen receptor α and inhibits calcification through the RANKL pathway,79,80 so the drop in estrogen in postmenopausal female individuals may increase RANKL activity and explain the limited calcifications observed in female patients with AS (Fig. 3). This modulation of the RANKL pathway may explain the cardioprotective role of estrogen in young female individuals, and the lack of calcification in young bicuspid female patients with AS.54
Notch
Notch is a transmembrane receptor protein involved in development, the differentiation of several cell types, and cardiac valvulogenesis.81 NOTCH1 mutations can cause developmental anomalies in the AV82,83 and are correlated with serum levels of OPG and RANKL,81 suggesting that Notch plays a role in valve calcification. Other studies suggest that Notch1 expression is decreased around calcification in AS, and that its downregulation in VICs increases calcification.84,85 A surprising finding is that a high level of expression of its ligand, Notch Delta-like 1, has been associated with mortality in patients suffering from symptomatic AS and osteogenic differentiation in myoblastic and osteoblastic cell lines.86,87 In VICs, increasing the expression of Notch1 decreases the expression of BMP2 and RUNX2 and prevents downstream mineralization (Fig. 3).88 Experiments in human endothelial kidney cells and osteoblastic lineages lead to the hypothesis that Notch1 repression may favour calcification in VECs and osteogenic differentiation in VICs.85,89,90 In regard to sex differences, sex-specific modulation of the Notch pathway in fibroblasts occurs in response to hyperoxia, but more studies are needed to highlight these differences in AS.91
TGF-β
The TGF-β signalling pathway has 2 major roles in AS—it is both proapoptotic and profibrotic.92, 93, 94, 95 TGF-β1 attenuates calcification and osteogenic differentiation of VICs in a 3-dimensional model of AS,96 and it favours cellular endothelial-mesenchymal transition.97 Very interesting data show that TGF-β induces calcification in VICs if they are grown on uncoated tissue culture plates, but calcification is repressed when cells are grown on fibronectin surfaces.98 These data mean that the TGF-β pathway in VICs may be sensitive to the microenvironment. BMPs belong to the TGF-β superfamily,99 but they have opposing roles, as BMPs are osteogenic growth factors promoting calcification in AS. BMPs are upregulated in AS patients and in animal models.100, 101, 102 For example, BMP-2 induces the expression of 2 pro-osteogenic factors, RUNX2 and SPP1, in human VICs.103 IL-6 promotes osteogenic differentiation of VICs and mineralization of the leaflets through the BMP-2 signalling pathway. 104 Like Notch1, BMP-2 is involved in embryonic cardiac valvular development.105
The balance between TGF-β and BMP signalling may differ in female and male AVs, and it could explain, at least in part, the sex-specific profibrotic and procalcific phenotypes observed.106 Only a few studies have addressed this regulation according to sex, but we know that the TGF-β signalling pathway is upregulated in female, compared to male, patients with AS. Three genes in this pathway, TGFβ2, MXRA5, and USP9X, are overexpressed in female individuals107: they may contribute to the accumulation of fibrosis in female patients. This hypothesis is corroborated by the fact that TGF-β2 has a profibrotic effect through the Suppressor of Mothers Against Decapentaplegic pathway and high levels of ECM proteins.108,109 TGF-β1 could have a different mechanism of action as it is overexpressed in calcifying conditions (male VICs and male AVs; Fig. 3).33,34 Sex-related mechanisms associated with TGF-β mainly are unknown, but genes associated with the BMP and/or TGF-β have been shown to regulate X-chromosome inactivation in female individuals.106,110
Wnt/β-catenin
The Wnt/β-catenin signalling pathway is involved in developmental processes, through regulation of cell proliferation and bone homeostasis.111 Low-density lipoprotein receptor-related protein 5 (LRP5), a co-receptor of Wnt, regulates the expression of bone matrix proteins in the AV and could modulate endochondral ossification in valvular heart diseases.102,112,113 β-catenin is overexpressed in stenotic AVs and is correlated with the pro-osteogenic response induced by MMP12 in VICs.114,115 The Wnt/β-catenin signalling pathway has been identified in AS, especially in its severe stages.115,116 This pathway also induces fibrosis in human aortic VICs (Fig. 3).117
We demonstrated that 4 genes in the Wnt pathway (DDX3X, GPC5, SFRP4, and LGR4) are upregulated in female patients with AS.107 An interesting finding is that estrogen receptor-1 modulates the expression of Wnt/β-catenin pathway genes, and estrogen receptor-α colocalizes with β-catenin in hepatocellular carcinoma.118 In adipocyte lineages, testosterone regulates the Wnt/β-catenin signalling pathway, suggesting a possible sex regulation of Wnt/β-catenin in AS.119 More studies are needed to determine how Wnt/β-catenin is regulated in female vs male individuals.
Sex hormones
Sex differences observed in AS may be associated with sex hormones. Circulating levels of testosterone have been correlated positively with vascular calcification, whereas serum levels of estrogen are associated negatively with vascular calcification.80,120,121 Estrogen has a protective effect against cardiac fibroblast proliferation, and testosterone attenuates myofibroblast activation.122,123 Androgen receptors and estrogen receptors type α and β are expressed in the AV, so they could play a key role in the sex differences found in AS.124
The underrepresentation of premenopausal female individuals in clinical studies slows the development of an understanding of estrogen’s effects on the cardiovascular system.80 Nevertheless, some studies show that estrogens exert their cardioprotective effect in premenopausal female individuals by modulating inflammation, lipoprotein metabolism, and vessel-wall properties.125,126 In postmenopausal female individuals, a polymorphism in the estrogen receptor-α has been correlated with the development of AS.127 Exposure to β-estradiol inhibits the proliferation of coronary smooth muscle cells in female, but not male, pigs.128 The influence of β-estradiol on VIC behaviour needs further study, but it seems to inhibit the proliferation of VICs in female, but not male, rats.32 An important finding is that 17β-estradiol inhibits the transcription of MMP2 through the mitogen-activated protein kinase extracellular signal-regulated kinase ½ (MAPK-ERK1/2) signalling pathway in cardiac fibroblasts, suggesting that estrogens play a role in ECM remodelling.44 17β-estradiol reduces fibrosis in female VICs only, but it does not affect calcification markers (BMP-4, RUNX2, osteopontin).57
The mechanism of action of androgens is poorly understood, but very interesting studies are emerging. First, Laukkanen et al. observed a correlation between high levels of testosterone and an increased risk of AS.129 Then, Fleury et al. showed that testosterone increases procalcific genes and worsens hemodynamic parameters in a male mouse model of the disease,130 but the direct mechanism of action of testosterone in the AV still is unknown. Testosterone may promote calcification of the AV via the androgen receptor, which is increased in stenotic AVs compared to normal AVs and is overexpressed in male, compared to female, individuals.124 An important point to note is that testosterone induces calcification in vascular smooth muscle cells through the androgen receptor,131 suggesting a similar mechanism of action in the AV, but the targeted cellular population still is unknown.
Genetic and Transcriptomic Studies Related to AS
Genetic factors are well known to be involved in the development of AS. Having a sibling with a history of AS increases the risk of having AS by a factor of 3, whereas having a spouse diagnosed with AS increases the risk of developing AS only slightly.132,133 Genetic factors seem to contribute more than environmental ones to the development of AS.134,135 LPA, PALMD, IL6, ALPL, NAV1, and TEX41 have been identified as susceptibility genes for AS, and single-nucleotide polymorphisms located near PALMD and IL1F9 are associated with AS.133, 134, 135, 136, 137, 138, 139 AS pathophysiology differs according to the sex of patients, so determining genetic risk factors specific to sex is important. This determination has been done with Lp(a), whose single-nucleotide variants are associated with high serum levels of Lp(a) and a higher risk of AS in both male and female individuals.140
AS also has been characterized at the transcriptomic level. In 2009, Bossé et al. showed that 715 genes were differently expressed in normal vs stenotic human AV.42 In healthy and stenotic valves, transcriptomic differences exist between male and female VICs and could explain the distinct VIC behaviour according to sex.141,142 Indeed, profibrotic processes seem to be upregulated in female individuals, whereas proinflammatory pathways seem to be overrepresented in male individuals.38 A recent study from our laboratory identified 190 genes that are expressed differently in female vs male patients with AS.107 Genes correlated with fibrosis were overexpressed in female patients (TGFβ2, KIF1A, FRAS1 . . .), whereas distinct genes linked to calcification were overexpressed in both female and male patients (female patients: RCN2; male patients: CPAMD8 and STC2).
Epigenetics might also be involved in the sex differences observed in AS, because sex is known to influence the expression of microRNA.143 Analysis has shown that 92 microRNAs are expressed differently in healthy vs stenotic AVs and may provide therapeutic targets for AS.144 Another microRNA analysis revealed that thrombospondin 1 (THBS1) and nuclear factor kappa beta (NFκB) inhibitor α (NFKBIA) are shear stress–sensitive genes in AV.145 However, this study does not describe male vs female differences.
Therapeutic Options and Biomarkers
Currently, no medical treatment is available to prevent or slow the progression of AS. When patients become symptomatic with severe AS, their prognosis is poor if they do not undergo surgical or transcatheter AV replacement.
The growing population with AS presents a critical need to develop medical treatments for AS, to reduce the burden of AV replacement and mortality. Several options have been tested, without success. Statins were proposed for AS treatment, owing to their efficacy in treating atherosclerosis. Several randomized controlled clinical trials using statins were conducted, but they did not slow the progression of AS.6,7,146 Thus, statins are not recommended for AS treatment.
High levels of Lp(a) are associated with AS progression and may identify patients who need early AV replacement.147 A therapy capable of reducing circulating Lp(a) levels may be promising for slowing AS progression. Pelacarsen (NCT05646381) and niacin (NCT02109614) currently are being tested in randomized controlled trials. Inclisiran has been shown to lower the low-density lipoprotein cholesterol level and the volume of Lp(a)-containing lipoprotein particles in atherosclerosis, so it would be interesting to test it in the context of AS.148
The RAAS is involved in fibrosis and AS progression, and it may be modulated in a sex-specific manner. In the Ramipril in Aortic Stenosis (RIAS) trial, an ACE inhibitor (Ramipril) led to a modest but progressive reduction of left ventricular mass in asymptomatic patients with moderate to severe AS, but it had no impact on AS progression.149 This result could be explained by secondary production of angiotensin II by chymase in the AV.50 The Angiotensin Receptor Blocker in Aortic Stenosis (ARBAS) trial (NCT04913870) is currently underway to test the efficacy of angiotensin-receptor blockers in patients with mild-to-moderate AS. Whether the results differ for treated female vs male patients will be interesting to see; the study has been designed to provide sex-specific results.
As osteogenic pathways contribute to AS progression, especially in male patients, therapies targeting these pathways also have been tested. Denosumab and bisphosphonates are 2 pharmacologic agents targeting the procalcific nuclear factor kappa beta (NFκB)/RANK/RANKL/OPG pathway. The Scottish Aortic Stenosis and Lipid Lowering Trial: Impact on Regression (SALTIRE) 2 trial showed no impact on reducing AV calcification or slowing AS hemodynamic progression.8
Although AS diagnosis can be performed easily by mostly noninvasive methods such as echocardiography, many patients remain undiagnosed or are diagnosed late in the course of the disease, especially female patients.11,12 Moreover, although the diagnosis of the disease is straightforward, evaluating its severity can be challenging,14 and predicting the progression rate for a given patient is almost impossible. Therefore, blood biomarkers associated with the valve lesions that are capable of raising suspicion, improving diagnosis rates, or predicting progression rates would be of major interest in identifying patients at risk sooner, and tailoring the timing of follow up. As Lp(a) is associated with the occurrence of AS, it is used to identify patients at risk of AS, but it also could be used as a biomarker to raise suspicion for AS. Unfortunately, studies considering sex differences in blood biomarkers are lacking, in terms of both sex-specific impact and sex-specific thresholds.
The only available biomarkers in current clinical practice, in addition to Lp(a), are those associated with the impact of AS on the ventricle, such as N-terminal pro-B type natriuretic peptide (NT-proBNP) or high-sensitivity troponin T. These biomarkers are useful in conducting risk stratification and determining intervention timing.150, 151, 152, 153, 154
Conclusion
Female patients present a more profibrotic remodelling of their stenosed AV, whereas male patients present more calcification. The pathophysiology of the disease is a complex phenomenon, orchestrated by several pathways in which sex-related specificities are certainly involved, but major knowledge gaps remain. However, the volume of evidence of sex-specific regulation of pathways is currently increasing.
Aldosterone increases markers of calcification in both sexes and fibrotic markers in female patients only. The RANK/RANKL/OPG pathway, which promotes calcification, seems to be upregulated in male patients with AS, compared to female patients. Regarding fibrosis, the TGF-β signalling pathway is upregulated in female patients, compared to male patients, with AS. Sex hormones seem to influence the phenotype of the VICs, with estrogen possibly inhibiting fibrosis, and testosterone promoting calcification and progression of AS. All these specificities may favour the fibrotic phenotype observed in female patients and the calcific phenotype observed in male patients with AS.
Aging is a risk factor for AS and a global characteristic of modern populations, so the need to develop medical therapies to slow or prevent AS is urgent. Given that the pathophysiology of AS differs according to sex, exploring sex-specific molecular mechanisms in AS is essential to developing sex-specific therapies.
Acknowledgements
All figures were created with BioRender.com.
Ethics Statement
The authors confirm that review by ethics committee was not applicable to this article, as no patient nor animal data were used for this article.
Patient Consent
The authors confirm that patient consent is not applicable to this article, as no patient data were used for this article.
Funding Sources
M.-A.C. holds the Canada Research Chair (CIHR: 950-232973) on Women’s Valvular Heart Health. All the other authors have no funding sources to declare.
Disclosures
M.-A.C. reports receiving funding from Edwards Lifesciences, Medtronic, and Pi-Cardia, with no direct personal compensation. All the other authors have no conflicts of interest to disclose.
Footnotes
See page 1133 for disclosure information.
References
- 1.Dweck M.R., Boon N.A., Newby D.E. Calcific aortic stenosis: a disease of the valve and the myocardium. J Am Coll Cardiol. 2012;60:1854–1863. doi: 10.1016/j.jacc.2012.02.093. [DOI] [PubMed] [Google Scholar]
- 2.Osnabrugge R.L., Mylotte D., Head S.J., et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. 2013;62:1002–1012. doi: 10.1016/j.jacc.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Yadgir S., Johnson C.O., Aboyans V., et al. Global, regional, and national burden of calcific aortic valve and degenerative mitral valve diseases, 1990–2017. Circulation. 2020;141:1670–1680. doi: 10.1161/CIRCULATIONAHA.119.043391. [DOI] [PubMed] [Google Scholar]
- 4.Frieden P., Blais C., Hamel D., et al. Evolution of the burden of aortic stenosis by sex in the province of Quebec between 2006 and 2018. Heart. 2022;108:1644–1650. doi: 10.1136/heartjnl-2021-319848. [DOI] [PubMed] [Google Scholar]
- 5.Otto C.M., Burwash I.G., Legget M.E., et al. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation. 1997;95:2262–2270. doi: 10.1161/01.cir.95.9.2262. [DOI] [PubMed] [Google Scholar]
- 6.Chan K.L., Teo K., Dumesnil J.G., Ni A., Tam J. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis. Results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. 2010;121:306–314. doi: 10.1161/CIRCULATIONAHA.109.900027. [DOI] [PubMed] [Google Scholar]
- 7.Rossebo A.B., Pedersen T.R., Boman K., et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 8.Pawade T.A., Doris M.K., Bing R., et al. Effect of denosumab or alendronic acid on the progression of aortic stenosis: a double-blind randomized controlled trial. Circulation. 2021;143:2418–2427. doi: 10.1161/CIRCULATIONAHA.121.053708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diederichsen A.C.P., Lindholt J.S., Möller S., et al. Vitamin K2 and D in patients with aortic valve calcification: a randomized double-blinded clinical trial. Circulation. 2022;145:1387–1397. doi: 10.1161/CIRCULATIONAHA.121.057008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bevan G.H., Zidar D.A., Josephson R.A., Al-Kindi S.G. Mortality due to aortic stenosis in the United States, 2008-2017. JAMA. 2019;321:2236–2238. doi: 10.1001/jama.2019.6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aggarwal S.R., Clavel M.A., Messika-Zeitoun D., et al. Sex differences in aortic valve calcification measured by multidetector computed tomography in aortic stenosis. Circ Cardiovasc Imaging. 2013;6:40–47. doi: 10.1161/CIRCIMAGING.112.980052. [DOI] [PubMed] [Google Scholar]
- 12.Simard L., Côté N., Dagenais F., et al. Sex-related discordance between aortic valve calcification and hemodynamic severity of aortic stenosis: Is valvular fibrosis the explanation? Circ Res. 2017;120:681–691. doi: 10.1161/CIRCRESAHA.116.309306. [DOI] [PubMed] [Google Scholar]
- 13.Capoulade R., Clavel M.A., Le Ven F., et al. Impact of left ventricular remodelling patterns on outcomes in patients with aortic stenosis. Eur Heart J Cardiovasc Imaging. 2017;18:1378–1387. doi: 10.1093/ehjci/jew288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clavel M.A., Burwash I.G., Pibarot P. Cardiac imaging for assessing low-gradient severe aortic stenosis. JACC Cardiovasc Imaging. 2017;10:185–202. doi: 10.1016/j.jcmg.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Deck J.D. Endothelial cell orientation on aortic valve leaflets. Cardiovasc Res. 1986;20:760–767. doi: 10.1093/cvr/20.10.760. [DOI] [PubMed] [Google Scholar]
- 16.Tseng H., Grande-Allen K.J. Elastic fibers in the aortic valve spongiosa: a fresh perspective on its structure and role in overall tissue function. Acta Biomater. 2011;7:2101–2108. doi: 10.1016/j.actbio.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shu L., Yuan Z., Li F., Cai Z. Oxidative stress and valvular endothelial cells in aortic valve calcification. Biomed Pharmacother. 2023;163 doi: 10.1016/j.biopha.2023.114775. [DOI] [PubMed] [Google Scholar]
- 18.Nishida K., Harrison D.G., Navas J.P., et al. Molecular cloning and characterization of the constitutive bovine aortic endothelial cell nitric oxide synthase. J Clin Invest. 1992;90:2092–2096. doi: 10.1172/JCI116092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hjortnaes J., Shapero K., Goettsch C., et al. Valvular interstitial cells suppress calcification of valvular endothelial cells. Atherosclerosis. 2015;242:251–260. doi: 10.1016/j.atherosclerosis.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu K., Xie S., Huang Y., et al. Cell-type transcriptome atlas of human aortic valves reveal cell heterogeneity and endothelial to mesenchymal transition involved in calcific aortic valve disease. Arterioscler Thromb Vasc Biol. 2020;40:2910–2921. doi: 10.1161/ATVBAHA.120.314789. [DOI] [PubMed] [Google Scholar]
- 21.Ma X., Zhao D., Yuan P., et al. Endothelial-to-mesenchymal transition in calcific aortic valve disease. Acta Cardiol Sin. 2020;36:183–194. doi: 10.6515/ACS.202005_36(3).20200213A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bosse K., Hans C.P., Zhao N., et al. Endothelial nitric oxide signaling regulates Notch1 in aortic valve disease. J Mol Cell Cardiol. 2013;60:27–35. doi: 10.1016/j.yjmcc.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson V., Patil V., Simon L.R., et al. Angiogenic secretion profile of valvular interstitial cells varies with cellular sex and phenotype. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.736303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu A.C., Joag V.R., Gotlieb A.I. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol. 2007;171:1407–1418. doi: 10.2353/ajpath.2007.070251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rutkovskiy A., Malashicheva A., Sullivan G., et al. Valve interstitial cells: the key to understanding the pathophysiology of heart valve calcification. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Visconti R.P., Ebihara Y., LaRue A.C., et al. An in vivo analysis of hematopoietic stem cell potential: hematopoietic origin of cardiac valve interstitial cells. Circ Res. 2006;98:690–696. doi: 10.1161/01.RES.0000207384.81818.d4. [DOI] [PubMed] [Google Scholar]
- 27.Butcher J.T., Markwald R.R. Valvulogenesis: the moving target. Philos Trans R Soc Lond B Biol Sci. 2007;362:1489–1503. doi: 10.1098/rstb.2007.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Rodriguez C., Parra-Izquierdo I., Castanos-Mollor I., et al. Toll-like receptors, inflammation, and calcific aortic valve disease. Front Physiol. 2018;9:201. doi: 10.3389/fphys.2018.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grim J.C., Aguado B.A., Vogt B.J., et al. Secreted factors from proinflammatory macrophages promote an osteoblast-like phenotype in valvular interstitial cells. Arterioscler Thromb Vasc Biol. 2020;40:e296–e308. doi: 10.1161/ATVBAHA.120.315261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J.H., Yip C.Y., Sone E.D., Simmons C.A. Identification and characterization of aortic valve mesenchymal progenitor cells with robust osteogenic calcification potential. Am J Pathol. 2009;174:1109–1119. doi: 10.2353/ajpath.2009.080750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bogdanova M., Zabirnyk A., Malashicheva A., et al. Interstitial cells in calcified aortic valves have reduced differentiation potential and stem cell-like properties. Sci Rep. 2019;9 doi: 10.1038/s41598-019-49016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masjedi S., Lei Y., Patel J., Ferdous Z. Sex-related differences in matrix remodeling and early osteogenic markers in aortic valvular interstitial cells. Heart Vessels. 2017;32:217–228. doi: 10.1007/s00380-016-0909-8. [DOI] [PubMed] [Google Scholar]
- 33.Simon L.R., Scott A.J., Figueroa Rios L., Zembles J., Masters K.S. Cellular-scale sex differences in extracellular matrix remodeling by valvular interstitial cells. Heart Vessels. 2023;38:122–130. doi: 10.1007/s00380-022-02164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matilla L., Martín-Núñez E., Garaikoetxea M., et al. Characterization of the sex-specific pattern of angiogenesis and lymphangiogenesis in aortic stenosis. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.971802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winchester R., Wiesendanger M., O'Brien W., et al. Circulating activated and effector memory T cells are associated with calcification and clonal expansions in bicuspid and tricuspid valves of calcific aortic stenosis. J Immunol. 2011;187:1006–1014. doi: 10.4049/jimmunol.1003521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hulin A., Hortells L., Gomez-Stallons M.V., et al. Maturation of heart valve cell populations during postnatal remodeling. Development. 2019;146 doi: 10.1242/dev.173047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Latif N., Sarathchandra P., Chester A.H., Yacoub M.H. Expression of smooth muscle cell markers and co-activators in calcified aortic valves. Eur Heart J. 2015;36:1335–1345. doi: 10.1093/eurheartj/eht547. [DOI] [PubMed] [Google Scholar]
- 38.Myasoedova V.A., Massaiu I., Moschetta D., et al. Sex-specific cell types and molecular pathways indicate fibro-calcific aortic valve stenosis. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.747714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parra-Izquierdo I., Castanos-Mollor I., Lopez J., et al. Calcification induced by type i interferon in human aortic valve interstitial cells is larger in males and blunted by a Janus kinase inhibitor. Arterioscler Thromb Vasc Biol. 2018;38:2148–2159. doi: 10.1161/ATVBAHA.118.311504. [DOI] [PubMed] [Google Scholar]
- 40.Aikawa E., Libby P. A rock and a hard place: chiseling away at the multiple mechanisms of aortic stenosis. Circulation. 2017;135:1951–1955. doi: 10.1161/CIRCULATIONAHA.117.027776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dahal S., Huang P., Murray B.T., Mahler G.J. Endothelial to mesenchymal transformation is induced by altered extracellular matrix in aortic valve endothelial cells. J Biomed Mater Res A. 2017;105:2729–2741. doi: 10.1002/jbm.a.36133. [DOI] [PubMed] [Google Scholar]
- 42.Bossé Y., Miqdad A., Fournier D., et al. Refining molecular pathways leading to calcific aortic valve stenosis by studying gene expression profile of normal and calcified stenotic human aortic valves. Circ Cardiovasc Genet. 2009;2:489–498. doi: 10.1161/CIRCGENETICS.108.820795. [DOI] [PubMed] [Google Scholar]
- 43.Yip C.Y., Chen J.H., Zhao R., Simmons C.A. Calcification by valve interstitial cells is regulated by the stiffness of the extracellular matrix. Arterioscler Thromb Vasc Biol. 2009;29:936–942. doi: 10.1161/ATVBAHA.108.182394. [DOI] [PubMed] [Google Scholar]
- 44.Mahmoodzadeh S., Dworatzek E., Fritschka S., Pham T.H., Regitz-Zagrosek V. 17beta-estradiol inhibits matrix metalloproteinase-2 transcription via MAP kinase in fibroblasts. Cardiovasc Res. 2010;85:719–728. doi: 10.1093/cvr/cvp350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conte M., Petraglia L., Campana P., et al. The role of inflammation and metabolic risk factors in the pathogenesis of calcific aortic valve stenosis. Aging Clin ExpRes. 2021;33:1765–1770. doi: 10.1007/s40520-020-01681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goody P.R., Hosen M.R., Christmann D., et al. Aortic valve stenosis: from basic mechanisms to novel therapeutic targets. Arterioscler Thromb Vasc Biol. 2020;40:885–900. doi: 10.1161/ATVBAHA.119.313067. [DOI] [PubMed] [Google Scholar]
- 47.Hajdu Z., Romeo S.J., Fleming P.A., et al. Recruitment of bone marrow-derived valve interstitial cells is a normal homeostatic process. J Mol Cell Cardiol. 2011;51:955–965. doi: 10.1016/j.yjmcc.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi J.H., Do Y., Cheong C., et al. Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. J Exp Med. 2009;206:497–505. doi: 10.1084/jem.20082129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mazzone A., Epistolato M.C., De Caterina R., et al. Neoangiogenesis, T-lymphocyte infiltration, and heat shock protein-60 are biological hallmarks of an immunomediated inflammatory process in end-stage calcified aortic valve stenosis. J Am Coll Cardiol. 2004;43:1670–1676. doi: 10.1016/j.jacc.2003.12.041. [DOI] [PubMed] [Google Scholar]
- 50.Helske S., Lindstedt K.A., Laine M., et al. Induction of local angiotensin II-producing systems in stenotic aortic valves. J Am Coll Cardiol. 2004;44:1859–1866. doi: 10.1016/j.jacc.2004.07.054. [DOI] [PubMed] [Google Scholar]
- 51.Lindman B.R., Clavel M.A., Mathieu P., et al. Calcific aortic stenosis. Nat Rev Dis Primers. 2016;2 doi: 10.1038/nrdp.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh S., Torzewski M. Fibroblasts and their pathological functions in the fibrosis of aortic valve sclerosis and atherosclerosis. Biomolecules. 2019;9:472. doi: 10.3390/biom9090472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liskova J., Hadraba D., Filova E., et al. Valve interstitial cell culture: production of mature type I collagen and precise detection. Microsc Res Tech. 2017;80:936–942. doi: 10.1002/jemt.22886. [DOI] [PubMed] [Google Scholar]
- 54.Voisine M., Hervault M., Shen M., et al. Age, sex, and valve phenotype differences in fibro-calcific remodeling of calcified aortic valve. J Am Heart Assoc. 2020 doi: 10.1161/JAHA.119.015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ames M.K., Atkins C.E., Pitt B. The renin-angiotensin-aldosterone system and its suppression. J Vet Intern Med. 2019;33:363–382. doi: 10.1111/jvim.15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tastet L., Capoulade R., Clavel M.A., et al. Systolic hypertension and progression of aortic valve calcification in patients with aortic stenosis: results from the PROGRESSA study. Eur Heart J Cardiovasc Imaging. 2017;18:70–78. doi: 10.1093/ehjci/jew013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matilla L., Jover E., Garaikoetxea M., et al. Sex-related signaling of aldosterone/mineralocorticoid receptor pathway in calcific aortic stenosis. Hypertension. 2022;79:1724–1737. doi: 10.1161/HYPERTENSIONAHA.122.19526. [DOI] [PubMed] [Google Scholar]
- 58.Medina D., Mehay D., Arnold A.C. Sex differences in cardiovascular actions of the renin-angiotensin system. Clin Auton Res. 2020;30:393–408. doi: 10.1007/s10286-020-00720-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schieffer B., Schieffer E., Hilfiker-Kleiner D., et al. Expression of angiotensin II and interleukin 6 in human coronary atherosclerotic plaques: potential implications for inflammation and plaque instability. Circulation. 2000;101:1372–1378. doi: 10.1161/01.cir.101.12.1372. [DOI] [PubMed] [Google Scholar]
- 60.Perez J., Diaz N., Tandon I., et al. Elevated serotonin interacts with angiotensin-II to result in altered valve interstitial cell contractility and remodeling. Cardiovasc Eng Technol. 2018;9:168–180. doi: 10.1007/s13239-017-0298-x. [DOI] [PubMed] [Google Scholar]
- 61.Kagami S., Border W.A., Miller D.E., Noble N.A. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-beta expression in rat glomerular mesangial cells. J Clin Invest. 1994;93:2431–2437. doi: 10.1172/JCI117251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Côté N., Pibarot P., Pépin A., et al. Oxidized low-density lipoprotein, angiotensin II and increased waist cirumference are associated with valve inflammation in prehypertensive patients with aortic stenosis. Int J Cardiol. 2010;145:444–449. doi: 10.1016/j.ijcard.2009.05.054. [DOI] [PubMed] [Google Scholar]
- 63.Yanes L.L., Romero D.G., Iles J.W., et al. Sexual dimorphism in the renin-angiotensin system in aging spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2006;291:R383–R390. doi: 10.1152/ajpregu.00510.2005. [DOI] [PubMed] [Google Scholar]
- 64.Nickenig G., Strehlow K., Wassmann S., et al. Differential effects of estrogen and progesterone on AT(1) receptor gene expression in vascular smooth muscle cells. Circulation. 2000;102:1828–1833. doi: 10.1161/01.cir.102.15.1828. [DOI] [PubMed] [Google Scholar]
- 65.Kjeldsen S.E. Hypertension and cardiovascular risk: general aspects. Pharmacol Res. 2018;129:95–99. doi: 10.1016/j.phrs.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 66.Xue B., Pamidimukkala J., Lubahn D.B., Hay M. Estrogen receptor-alpha mediates estrogen protection from angiotensin II-induced hypertension in conscious female mice. Am J Physiol Heart Circ Physiol. 2007;292:H1770–H1776. doi: 10.1152/ajpheart.01011.2005. [DOI] [PubMed] [Google Scholar]
- 67.Fischer M., Baessler A., Schunkert H. Renin angiotensin system and gender differences in the cardiovascular system. Cardiovasc Res. 2002;53:672–677. doi: 10.1016/s0008-6363(01)00479-5. [DOI] [PubMed] [Google Scholar]
- 68.Colafella K.M.M., Denton K.M. Sex-specific differences in hypertension and associated cardiovascular disease. Nat Rev Nephrol. 2018;14:185–201. doi: 10.1038/nrneph.2017.189. [DOI] [PubMed] [Google Scholar]
- 69.Gersh F.L., O'Keefe J.H., Lavie C.J., Henry B.M. The renin-angiotensin-aldosterone system in postmenopausal women: the promise of hormone therapy. Mayo Clin Proc. 2021;96:3130–3141. doi: 10.1016/j.mayocp.2021.08.009. [DOI] [PubMed] [Google Scholar]
- 70.Komukai K., Mochizuki S., Yoshimura M. Gender and the renin-angiotensin-aldosterone system. Fundam Clin Pharmacol. 2010;24:687–698. doi: 10.1111/j.1472-8206.2010.00854.x. [DOI] [PubMed] [Google Scholar]
- 71.Tatchum-Talom R., Eyster K.M., Martin D.S. Sexual dimorphism in angiotensin II-induced hypertension and vascular alterations. Can J Physiol Pharmacol. 2005;83:413–422. doi: 10.1139/y05-012. [DOI] [PubMed] [Google Scholar]
- 72.Akahori H., Tsujino T., Masuyama T., Ishihara M. Mechanisms of aortic stenosis. J Cardiol. 2018;71:215–220. doi: 10.1016/j.jjcc.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 73.Pawade T.A., Newby D.E., Dweck M.R. Calcification in aortic stenosis: the skeleton key. J Am Coll Cardiol. 2015;66:561–577. doi: 10.1016/j.jacc.2015.05.066. [DOI] [PubMed] [Google Scholar]
- 74.Phua K., Chew N.W., Kong W.K., et al. The mechanistic pathways of oxidative stress in aortic stenosis and clinical implications. Theranostics. 2022;12:5189–5203. doi: 10.7150/thno.71813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaden J.J., Bickelhaupt S., Grobholz R., et al. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulate aortic valve calcification. J Mol Cell Cardiol. 2004;36:57–66. doi: 10.1016/j.yjmcc.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 76.Byon C.H., Sun Y., Chen J., et al. Runx2-upregulated receptor activator of nuclear factor kappaB ligand in calcifying smooth muscle cells promotes migration and osteoclastic differentiation of macrophages. Arterioscler Thromb Vasc Biol. 2011;31:1387–1396. doi: 10.1161/ATVBAHA.110.222547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matilla L., Garaikoetxea M., Arrieta V., et al. Sex-differences in aortic stenosis: mechanistic insights and clinical implications. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.818371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Simard L., Dagenais F., Pibarot P., et al. Impact of sex on aortic valve calcification and fibrosis in aortic stenosis. Can J Cardiol. 2015;31:S312–S313. [Google Scholar]
- 79.Osako M.K., Nakagami H., Koibuchi N., et al. Estrogen inhibits vascular calcification via vascular RANKL system: common mechanism of osteoporosis and vascular calcification. Circ Res. 2010;107:466–475. doi: 10.1161/CIRCRESAHA.110.216846. [DOI] [PubMed] [Google Scholar]
- 80.Woodward H.J., Zhu D., Hadoke P.W.F., MacRae V.E. Regulatory role of sex hormones in cardiovascular calcification. Int J Molec Sci. 2021;22:4620. doi: 10.3390/ijms22094620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Irtyuga O., Malashicheva A., Zhiduleva E., et al. NOTCH1 mutations in aortic stenosis: association with osteoprotegerin/RANK/RANKL. BioMed Res Int. 2017;2017:1–10. doi: 10.1155/2017/6917907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Garg V., Muth A.N., Ransom J.F., et al. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437:270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 83.Theodoris C.V., Li M., White M.P., et al. Human disease modeling reveals integrated transcriptional and epigenetic mechanisms of NOTCH1 haploinsufficiency. Cell. 2015;160:1072–1086. doi: 10.1016/j.cell.2015.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Acharya A., Hans C.P., Koenig S.N., et al. Inhibitory role of Notch1 in calcific aortic valve disease. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0027743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nigam V., Srivastava D. Notch1 represses osteogenic pathways in aortic valve cells. J Mol Cell Cardiol. 2009;47:828–834. doi: 10.1016/j.yjmcc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abraityte A., Gullestad L., Askevold E.T., et al. The Notch ligand Delta-like 1 is elevated and associated with mortality in patients with symptomatic aortic stenosis. Int J Cardiol. 2015;180:18–20. doi: 10.1016/j.ijcard.2014.11.111. [DOI] [PubMed] [Google Scholar]
- 87.Nobta M., Tsukazaki T., Shibata Y., et al. Critical regulation of bone morphogenetic protein-induced osteoblastic differentiation by Delta1/Jagged1-activated Notch1 signaling. J Biol Chem. 2005;280:15842–15848. doi: 10.1074/jbc.M412891200. [DOI] [PubMed] [Google Scholar]
- 88.Hadji F., Boulanger M.-C., Guay S.-P., et al. Altered DNA methylation of long noncoding RNA H19 in calcific aortic valve disease promotes mineralization by silencing NOTCH1. Circulation. 2016;134:1848–1862. doi: 10.1161/CIRCULATIONAHA.116.023116. [DOI] [PubMed] [Google Scholar]
- 89.Miller J.D., Weiss R.M., Heistad D.D. Calcific aortic valve stenosis: methods, models, and mechanisms. Circ Res. 2011;108:1392–1412. doi: 10.1161/CIRCRESAHA.110.234138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ann E.-J., Kim H.-Y., Choi Y.-H., et al. Inhibition of Notch1 signaling by Runx2 during osteoblast differentiation. J Bone Miner Res. 2011;26:317–330. doi: 10.1002/jbmr.227. [DOI] [PubMed] [Google Scholar]
- 91.Balaji S., Dong X., Li H., et al. Sex-specific differences in primary neonatal murine lung fibroblasts exposed to hyperoxia in vitro: implications for bronchopulmonary dysplasia. Physiol Genomics. 2018;50:940–946. doi: 10.1152/physiolgenomics.00075.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu A.C., Gotlieb A.I. Transforming growth factor-beta regulates in vitro heart valve repair by activated valve interstitial cells. Am J Pathol. 2008;173:1275–1285. doi: 10.2353/ajpath.2008.080365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jian B., Narula N., Li Q.Y., Mohler E.R., 3rd, Levy R.J. Progression of aortic valve stenosis: TGF-beta1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann Thorac Surg. 2003;75:457–465. doi: 10.1016/s0003-4975(02)04312-6. [DOI] [PubMed] [Google Scholar]
- 94.Shanker G., Olson D., Bone R., Sawhney R. Regulation of extracellular matrix proteins by transforming growth factor beta1 in cultured pulmonary endothelial cells. Cell Biol Int. 1999;23:61–72. doi: 10.1006/cbir.1998.0325. [DOI] [PubMed] [Google Scholar]
- 95.Hinz B. The extracellular matrix and transforming growth factor-beta1: tale of a strained relationship. Matrix Biol. 2015;47:54–65. doi: 10.1016/j.matbio.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 96.Jenke A., Kistner J., Saradar S., et al. Transforming growth factor-beta1 promotes fibrosis but attenuates calcification of valvular tissue applied as a three-dimensional calcific aortic valve disease model. Am J Physiol Heart Circ Physiol. 2020;319:H1123–H1141. doi: 10.1152/ajpheart.00651.2019. [DOI] [PubMed] [Google Scholar]
- 97.Paranya G., Vineberg S., Dvorin E., et al. Aortic valve endothelial cells undergo transforming growth factor-beta-mediated and non-transforming growth factor-beta-mediated transdifferentiation in vitro. Am J Pathol. 2001;159:1335–1343. doi: 10.1016/s0002-9440(10)62520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Benton J.A., Kern H.B., Anseth K.S. Substrate properties influence calcification in valvular interstitial cell culture. J Heart Valve Dis. 2008;17:689–699. [PMC free article] [PubMed] [Google Scholar]
- 99.Wrana J.L. Signaling by the TGFbeta superfamily. Cold Spring Harb Perspect Biol. 2013;5 doi: 10.1101/cshperspect.a011197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ankeny R.F., Thourani V.H., Weiss D., et al. Preferential activation of SMAD1/5/8 on the fibrosa endothelium in calcified human aortic valves—association with low BMP antagonists and SMAD6. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Seya K., Yu Z., Kanemaru K., et al. Contribution of bone morphogenetic protein-2 to aortic valve calcification in aged rat. J Pharmacol Sci. 2011;115:8–14. doi: 10.1254/jphs.10198fp. [DOI] [PubMed] [Google Scholar]
- 102.Miller J.D., Weiss R.M., Serrano K.M., et al. Evidence for active regulation of pro-osteogenic signaling in advanced aortic valve disease. Arterioscler Thromb Vasc Biol. 2010;30:2482–2486. doi: 10.1161/ATVBAHA.110.211029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang X., Meng X., Su X., et al. Bone morphogenic protein 2 induces Runx2 and osteopontin expression in human aortic valve interstitial cells: role of Smad1 and extracellular signal-regulated kinase 1/2. J Thorac Cardiovasc Surg. 2009;138:1008–1015. doi: 10.1016/j.jtcvs.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 104.El Husseini D., Boulanger M.C., Mahmut A., et al. P2Y2 receptor represses IL-6 expression by valve interstitial cells through Akt: implication for calcific aortic valve disease. J Mol Cell Cardiol. 2014;72:146–156. doi: 10.1016/j.yjmcc.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 105.Papoutsi T., Luna-Zurita L., Prados B., Zaffran S., de la Pompa J.L. Bmp2 and Notch cooperate to pattern the embryonic endocardium. Development. 2018;145 doi: 10.1242/dev.163378. [DOI] [PubMed] [Google Scholar]
- 106.Shah T.A., Rogers M.B. Unanswered questions regarding sex and BMP/TGF-β signaling. J Dev Biol. 2018;6:14. doi: 10.3390/jdb6020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Le Nezet E., Voisine M., Tam R., et al. 2023. Transcriptomic analysis reveals sex differences in gene expression profiling of stenotic aortic valves. Paper presented at: American Heart Association scientific sessions. xxx. Philadelphia, PA. [Google Scholar]
- 108.Frangogiannis N.G. Cardiac fibrosis: cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol Aspects Med. 2019;65:70–99. doi: 10.1016/j.mam.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 109.Zode G.S., Sethi A., Brun-Zinkernagel A.M., et al. Transforming growth factor-beta2 increases extracellular matrix proteins in optic nerve head cells via activation of the Smad signaling pathway. Mol Vis. 2011;17:1745–1758. [PMC free article] [PubMed] [Google Scholar]
- 110.Sripathy S., Leko V., Adrianse R.L., et al. Screen for reactivation of MeCP2 on the inactive X chromosome identifies the BMP/TGF-β superfamily as a regulator of XIST expression. Proc Natl Acad Sci U S A. 2017;114:1619–1624. doi: 10.1073/pnas.1621356114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rim E.Y., Clevers H., Nusse R. The Wnt pathway: from signaling mechanisms to synthetic modulators. Annu Rev Biochem. 2022;91:571–598. doi: 10.1146/annurev-biochem-040320-103615. [DOI] [PubMed] [Google Scholar]
- 112.Rajamannan N.M., Subramaniam M., Caira F., Stock S.R., Spelsberg T.C. Atorvastatin inhibits hypercholesterolemia-induced calcification in the aortic valves via the Lrp5 receptor pathway. Circulation. 2005;112 doi: 10.1161/01.CIRCULATIONAHA.104.524306. I-229-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fang M., Wang C.G., Zheng C., et al. Mir-29b promotes human aortic valve interstitial cell calcification via inhibiting TGF-beta3 through activation of wnt3/beta-catenin/Smad3 signaling. J Cell Biochem. 2018;119:5175–5185. doi: 10.1002/jcb.26545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Deng X.S., Meng X., Li F., et al. MMP-12-induced pro-osteogenic responses in human aortic valve interstitial cells. J Surg Res. 2019;235:44–51. doi: 10.1016/j.jss.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Khan K., Yu B., Kiwan C., et al. The role of Wnt/β-catenin pathway mediators in aortic valve stenosis. Front Cell Dev Biol. 2020;8:862. doi: 10.3389/fcell.2020.00862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhong A., Mirzaei Z., Simmons C.A. The roles of matrix stiffness and ss-catenin signaling in endothelial-to-mesenchymal transition of aortic valve endothelial cells. Cardiovasc Eng Technol. 2018;9:158–167. doi: 10.1007/s13239-018-0363-0. [DOI] [PubMed] [Google Scholar]
- 117.Jarrett M.J., Houk A.K., McCuistion P.E., et al. Wnt signaling mediates pro-fibrogenic activity in human aortic valve interstitial cells. Ann Thorac Surg. 2021;112:519–525. doi: 10.1016/j.athoracsur.2020.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bhat M., Pasini E., Pastrello C., et al. Estrogen receptor 1 inhibition of Wnt/beta-catenin signaling contributes to sex differences in hepatocarcinogenesis. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.777834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Singh R., Artaza J.N., Taylor W.E., et al. Testosterone inhibits adipogenic differentiation in 3T3-L1 cells: nuclear translocation of androgen receptor complex with beta-catenin and T-cell factor 4 may bypass canonical Wnt signaling to down-regulate adipogenic transcription factors. Endocrinology. 2006;147:141–154. doi: 10.1210/en.2004-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nakao J., Orimo H., Ooyama T., Shiraki M. Low serum estradiol levels in subjects with arterial calcification. Atherosclerosis. 1979;34:469–474. doi: 10.1016/0021-9150(79)90071-6. [DOI] [PubMed] [Google Scholar]
- 121.Pérez-López F.R., Larrad-Mur L., Kallen A., Chedraui P., Taylor H.S. Gender differences in cardiovascular disease: hormonal and biochemical influences. Reprod Sci. 2010;17:511–531. doi: 10.1177/1933719110367829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dubey R.K., Gillespie D.G., Jackson E.K., Keller P.J. 17β-estradiol, its metabolites, and progesterone inhibit cardiac fibroblast growth. Hypertension. 1998;31:522–528. doi: 10.1161/01.hyp.31.1.522. [DOI] [PubMed] [Google Scholar]
- 123.Chung C.C., Hsu R.C., Kao Y.H., et al. Androgen attenuates cardiac fibroblasts activations through modulations of transforming growth factor-beta and angiotensin II signaling. Int J Cardiol. 2014;176:386–393. doi: 10.1016/j.ijcard.2014.07.077. [DOI] [PubMed] [Google Scholar]
- 124.Eildermann K., Goldmann S., Krause U., et al. Differences in androgen receptor expression in human heart tissue in various types of cardiomyopathy and in aortic valve stenosis. J Cardiovasc Dev Dis. 2023;10:466. doi: 10.3390/jcdd10110466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Farhat M.Y., Lavigne M.C., Ramwell P.W. The vascular protective effects of estrogen. FASEB J. 1996;10:615–624. [PubMed] [Google Scholar]
- 126.Chakrabarti S., Lekontseva O., Davidge S.T. Estrogen is a modulator of vascular inflammation. IUBMB Life. 2008;60:376–382. doi: 10.1002/iub.48. [DOI] [PubMed] [Google Scholar]
- 127.Nordstrom P., Glader C.A., Dahlen G., et al. Oestrogen receptor alpha gene polymorphism is related to aortic valve sclerosis in postmenopausal women. J Intern Med. 2003;254:140–146. doi: 10.1046/j.1365-2796.2003.01179.x. [DOI] [PubMed] [Google Scholar]
- 128.Fitzpatrick L.A. Gender-related differences in the development of atherosclerosis: studies at the cellular level. Clin Exp Pharmacol Physiol. 1996;23:267–269. doi: 10.1111/j.1440-1681.1996.tb02609.x. [DOI] [PubMed] [Google Scholar]
- 129.Laukkanen J.A., Kurl S., Kunutsor S.K. Cardiorespiratory fitness and risk of aortic stenosis (from a prospective cohort analysis) Am J Cardiol. 2023;201:101–106. doi: 10.1016/j.amjcard.2023.05.065. [DOI] [PubMed] [Google Scholar]
- 130.Fleury M.A., Annabi M.S., Voisine M., et al. Impact of sex and sex hormones on pathophysiology and progression of aortic stenosis in a murine model. Physiol Rep. 2022;10 doi: 10.14814/phy2.15433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhu D., Hadoke P.W., Wu J., et al. Ablation of the androgen receptor from vascular smooth muscle cells demonstrates a role for testosterone in vascular calcification. Sci Rep. 2016;6 doi: 10.1038/srep24807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Martinsson A., Li X., Zöller B., et al. Familial aggregation of aortic valvular stenosis: a nationwide study of sibling risk. Circ Cardiovasc Genet. 2017;10 doi: 10.1161/CIRCGENETICS.117.001742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Moncla L.M., Briend M., Bossé Y., Mathieu P. Calcific aortic valve disease: mechanisms, prevention and treatment. Nat Rev Cardiol. 2023;20:546–559. doi: 10.1038/s41569-023-00845-7. [DOI] [PubMed] [Google Scholar]
- 134.Thériault S., Gaudreault N., Lamontagne M., et al. A transcriptome-wide association study identifies PALMD as a susceptibility gene for calcific aortic valve stenosis. Nat Commun. 2018;9:998. doi: 10.1038/s41467-018-03260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Thériault S., Dina C., Messika-Zeitoun D., et al. Genetic association analyses highlight IL6, ALPL, and NAV1 as 3 new susceptibility genes underlying calcific aortic valve stenosis. Circ Genom Precis Med. 2019;12 doi: 10.1161/CIRCGEN.119.002617. [DOI] [PubMed] [Google Scholar]
- 136.Thanassoulis G., Campbell C.Y., Owens D.S., et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503–512. doi: 10.1056/NEJMoa1109034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Helgadottir A., Thorleifsson G., Gretarsdottir S., et al. Genome-wide analysis yields new loci associating with aortic valve stenosis. Nat Commun. 2018;9:987. doi: 10.1038/s41467-018-03252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Trenkwalder T., Nelson C.P., Musameh M.D., et al. Effects of the coronary artery disease associated LPA and 9p21 loci on risk of aortic valve stenosis. Int J Cardiol. 2019;276:212–217. doi: 10.1016/j.ijcard.2018.11.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen H.Y., Dufresne L., Burr H., et al. Association of LPA variants with aortic stenosis: a large-scale study using diagnostic and procedural codes from electronic health records. JAMA Cardiol. 2018;3:18–23. doi: 10.1001/jamacardio.2017.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Guertin J., Kaiser Y., Manikpurage H., et al. Sex-specific associations of genetically-predicted circulating lipoprotein(a) and hepatic LPA gene expression levels with cardiovascular outcomes: mendelian randomization and observational analyses. Circ Genom Precis Med. 2021;14 doi: 10.1161/CIRCGEN.120.003271. [DOI] [PubMed] [Google Scholar]
- 141.McCoy C.M., Nicholas D.Q., Masters K.S. Sex-related differences in gene expression by porcine aortic valvular interstitial cells. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sarajlic P., Plunde O., Franco-Cereceda A., Bäck M. Artificial intelligence models reveal sex-specific gene expression in aortic valve calcification. JACC Basic Transl Sci. 2021;6:403–412. doi: 10.1016/j.jacbts.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sharma S., Eghbali M. Influence of sex differences on microRNA gene regulation in disease. Biol Sex Differ. 2014;5:3. doi: 10.1186/2042-6410-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang H., Shi J., Li B., et al. MicroRNA expression signature in human calcific aortic valve disease. BioMed Res Int. 2017;2017 doi: 10.1155/2017/4820275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Holliday C.J., Ankeny R.F., Jo H., Nerem R.M. Discovery of shear- and side-specific mRNAs and miRNAs in human aortic valvular endothelial cells. Am J Physiol Heart Circ Physiol. 2011;301:H856–H867. doi: 10.1152/ajpheart.00117.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cowell S.J., Newby D.E., Prescott R.J., et al. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352:2389–2397. doi: 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- 147.Capoulade R., Chan K.L., Yeang C., et al. Oxidized phospholipids, lipoprotein(a), and progression of calcific aortic valve stenosis. J Am Coll Cardiol. 2015;66:1236–1246. doi: 10.1016/j.jacc.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 148.Ray K.K., Wright R.S., Kallend D., et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382:1507–1519. doi: 10.1056/NEJMoa1912387. [DOI] [PubMed] [Google Scholar]
- 149.Bull S., Loudon M., Francis J.M., et al. A prospective, double-blind, randomized controlled trial of the angiotensin-converting enzyme inhibitor Ramipril in aortic stenosis (RIAS trial) Eur Heart J Cardiovasc Imaging. 2015;16:834–841. doi: 10.1093/ehjci/jev043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hadziselimovic E., Greve A.M., Sajadieh A., et al. Association of annual N-terminal pro-brain natriuretic peptide measurements with clinical events in patients with asymptomatic nonsevere aortic stenosis: a post hoc substudy of the SEAS trial. JAMA Cardiol. 2022;7:435–444. doi: 10.1001/jamacardio.2021.5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Holmgren A., Ljungberg J., Hultdin J., et al. Troponin T but not C reactive protein is associated with future surgery for aortic stenosis: a population-based nested case-referent study. Open Heart. 2020;7 doi: 10.1136/openhrt-2020-001325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Olgun Kucuk H., Kucuk U., Demirtas C., Ozdemir M. Role of serum high density lipoprotein levels and functions in calcific aortic valve stenosis progression. Int J Clin Exp Med. 2015;8:22543–22549. [PMC free article] [PubMed] [Google Scholar]
- 153.White M., Baral R., Ryding A., et al. Biomarkers associated with mortality in aortic stenosis: a systematic review and meta-analysis. Med Sci (Basel) 2021;9:29. doi: 10.3390/medsci9020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sarkar A., Chowdhury S., Kumar A., et al. Biomarkers as prognostic markers for aortic stenosis: a review. Am J Cardiol. 2023;206:53–59. doi: 10.1016/j.amjcard.2023.08.001. [DOI] [PubMed] [Google Scholar]