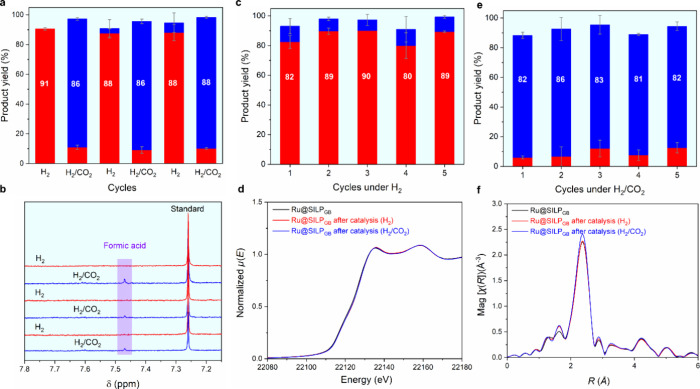
Figure 6.
(a) Product distributions for consecutive cycles of the hydrogenation of 1 using Ru@SILPGB while alternating the feed gas between H2 and H2/CO2 and (b) 1H NMR monitoring of the HCOOH content of the reaction solutions as a function of the feed gas composition. (c, e) Recycling experiments for the hydrogenation of furfuralacetone (1) using Ru@SILPGB under (c) H2 and (e) H2/CO2; corresponding (d) k2-weighted R-space FT-EXAFS spectra and (f) Ru K-edge XANES spectra (normalized) plot in Magnitude without phase correction) for Ru@SILPGB after reaction under H2 and H2/CO2. Reaction conditions: Ru@SILPGB (20 mg, 0.007 mmol Ru), furfuralacetone (1, 0.25 mmol, 35 equiv), 1,4-dioxane (1 mL), H2 (15 bar) or H2/CO2 (45 bar, 1:2), 80 °C, 16 h for (a, b), 2 h for (c,e). Product yield determined by GC-FID using tetradecane as the internal standard. Conversion >99% and the byproduct is 2,2′-(oxybis(butane-3,1-diyl))bis(tetrahydrofuran). Data points are average values of three experiments and error bars represent standard deviations. Red bars: product 1d; blue bars: product 1b.