FIGURE 6.
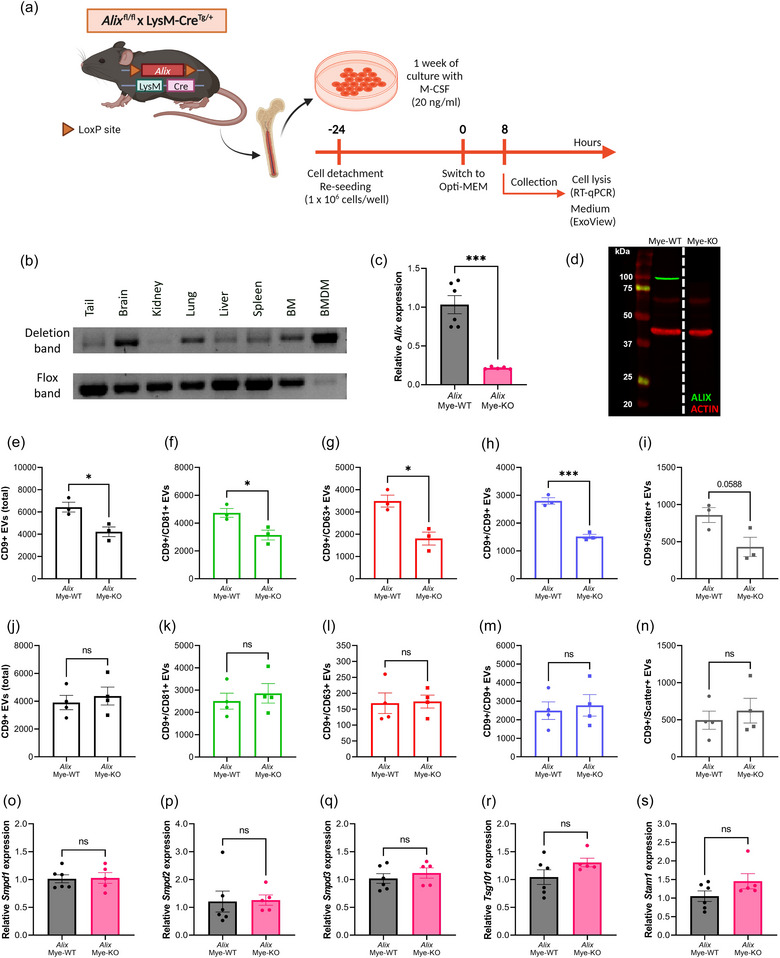
Alix deletion in bone marrow‐derived macrophages (BMDMs) results in decreased EV production. (a) BMDMs were differentiated from whole bone marrow from Alix fl/fl × LysM‐CreTg/+ (Alix Mye‐KO) mice and Alix fl/fl × LysM‐Cre+/+ (Alix Mye‐WT). At 24 h before the start of the assays, BMDMs were re‐seeded at equal cell densities. At the start of incubation, the culture medium was switched to Opti‐MEM. Cells were lysed and culture medium was collected for analyses 8 h after switching to Opti‐MEM medium. (b) Deflox PCR on several myeloid cell‐containing organs and BMDMs derived from AlixMye‐KO mice. The presence of the deletion band (473 bp) shows effective cre‐mediated ‘defloxing’. The flox band (368 bp) is merely absent in BMDMs while still present in the organs. (c) Alix expression in BMDMs analysed with RT‐qPCR, relative to AlixMye‐WT, showing efficient KO of Alix on RNA level. (d) ALIX western blot, visualizing the absence of ALIX (96 kDa) in BMDMs from AlixMye‐KO BMDM lysates. Red bands represent ACTIN protein (42 kDa) expression. (e–n) ExoView quantification of CD9+ EVs in BMDM culture medium (e–i) and plasma (j–n) from AlixMye‐WT (n = 3–4) and AlixMye‐KO (n = 3–4) mice, showing the total (e, j), CD81+ (f, k), CD63+ (g, l), CD9+ (h, m) and label‐free scatter+ (50–200 nm) (i, n) EVs captured on the CD9 spot. (O–S) Expression level of Smpd1 (o), Smpd2 (p), Smpd3 (q), Tsg101 (r) and Stam1 (s) in BMDMs from AlixMye‐WT (n = 6) versus AlixMye‐KO mice (n = 5). Results are represented relative to AlixMye‐WT. Data are represented as means ± SEM. Statistical analyses were performed by unpaired t‐testing or Mann–Whitney testing (*p < 0.05, ***p < 0.001, ns, not significant). Alix, apoptosis linked gene 2 interacting protein X; BMDM, bone marrow‐derived macrophage; EV, extracellular vesicle; Smpd, sphingomyelin phosphodiesterase.
