Abstract
Historically marginalized communities are disproportionately affected by cardiometabolic diseases yet are underrepresented in clinical trials that investigate needed interventions. This review investigates the barriers to equitable inclusion in clinical trials, identifying opportunities for improvement at the institutional, trial, community, and individual level. It proposes a social determinants-based approach that serves as a toolkit to target these barriers using structural, economic, community, healthcare access, and technology solutions, supporting constructive improvement in the clinical trial recruitment process.
Keywords: underrepresentation, clinical trials, recruitment, social determinants
Introduction
Clinical trials are the cornerstone of medical research and provide practice-changing evidence to recommend interventions that work best to improve patient outcomes.1 However, the participants of clinical trials often fail to adequately include populations most impacted by chronic diseases. These include underrepresented groups defined by gender identity, sexual orientation, ethnicity, race, socioeconomic status, and disability, among others.2 Of the 55 drugs approved by the US Food and Drug Administration (FDA) in 2023, only nine drug trials enrolled at least 10% of individuals identifying as Black race despite comprising 12% of the United States population.3,4 Similarly, those of Asian, Hispanic, American Indian or Alaskan Native descent were enrolled significantly less than their population prevalence.3,4 Gender minorities are rarely reported in clinical trials (0.1% of trials in one estimate), and those with intellectual or physical disability are often excluded from participation.5,6 Finally, in cardiovascular medicine, studies have found that race is reported in only 23% of clinical trials and, when reported, these groups are vastly underrepresented compared to their population estimates.7,8 This underrepresentation threatens external validity and precludes benefit for these communities, who carry a disproportionate burden of disease. Recent attempts have been made to improve representation in clinical trials, identifying structural, social, and economic barriers for individuals and their communities but also identifying similar barriers inherent in current trial design on an institutional level.2,9,10 Thus, the prominence of the social determinants of health in clinical trials—namely economic stability, education access and quality, healthcare access, social and community context, and neighborhood environment—cannot be understated. This review summarizes key issues regarding recruitment of underrepresented groups in clinical trials, with a particular focus on the social determinants of health from the institutional level to the individual level, and provides proven and burgeoning processes to combat recruitment barriers.
Barriers
Institutional and Trial Level
Clinical trials require a scientific question, study population, research site, protocol, and funding. Research questions are developed by scientists, whose interests may not fully reflect the needs of underrepresented groups.9 Research questions are also often driven by funding opportunities, narrowing their scope to fulfill investor expectations. In recent years, industry-funded trials have skyrocketed while government-funded trials have decreased.11 Industry funding alone has been associated with reduced representation, including minority race and ethnic groups.12,13,14 Further disparities exist within the exclusion criteria used to select the trial population itself, as underrepresented groups often fail to meet eligibility criteria.15,16,17,18,19 Reasons cited include lack of preexisting data, advanced disease at presentation, and comorbidities, all of which relate to inequities in healthcare access and treatment. Inclusion criteria may also promote under-enrollment, such as in heart failure trials that establish eligibility using natriuretic peptides even though there are racial differences in baseline levels, likely related to access to care and chronic disease management.20,21 Research sites and recruitment are often at large academic medical institutions, leading to lack of geographic and rural diversity as well as lack of socioeconomic, racial, and ethnic diversity.22,23,24,25 If there is recruitment outside of the medical institution, it may be through a website or journal that does not reach underrepresented groups.9 Protocol development includes the language and words used in all materials of the study, including recruitment material, consent forms, and qualitative surveys, which can be inadequate for the nuanced dialect and literacy level of participants from underrepresented groups.25,26,27 In addition, trial protocols often place undue burden on participants, requiring multiple clinic visits or laboratory draws, without patient-centric end points.28
Underrepresentation is prevalent among trial investigators as well, widening dissimilarities between participants and research. Less than 10% of pharmaceutical and biotechnology CEOs are female, and there are significantly less principal investigators of racial and ethnic minorities.29,30 While seemingly distant from trial participants, studies have shown associations between trial leadership and congruent recruitment. For instance, cardiovascular clinical trials with female principal investigators and a greater number of women authors were associated with higher enrollment of women participants.31,32 Trial staff, such as those involved in enrollment, clinic site visits, and data collection, also play roles in trial perceptions and concordant care.9,33
Community Level
The community context of underrepresented groups is an important social determinant for study recruitment. At the community level, engagement of stakeholders by clinical trials is often suboptimal, leading to decreased recruitment of underrepresented groups. Time and budget constraints and stakeholder unawareness of trial opportunities were commonly cited barriers to stakeholder engagement.9,10 Stakeholders may include community primary care physicians, who may be unable to support clinical trial recruitment due to structural and regulatory challenges and the impact on workload and productivity.34 Finally, there may be mistrust in certain groups rooted in structural discriminatory practices in research and science, although recent studies suggest that this barrier can be overcome through targeted outreach and community engagement.35,36
Individual Level
There are several barriers at the individual level for clinical trial participation, including economic, environmental, and healthcare access. While cited at the individual level, these barriers are a product of systemic inequities. Women, Black, Hispanic, and disabled individuals have consistently lower median incomes and less paid leave than their white, male counterparts.37,38,39 Time and even the cost of transportation can be significant barriers to trial participation, particularly when considering the loss of potential wages.9,10 Unstable housing, more common among historically marginalized groups, can lead to frequent moves and loss of trial follow-up.40 Similarly, reliable telephone access constitutes another barrier for individual enrollment and drop out.40 Finally, regular access to a primary care provider and satisfaction with health care (in terms of knowledge, trust, and perception) are significant reasons an individual may or may not participate in a trial.9,41,42
Proposed Solutions
Structural
For trial design, several structural changes have been posited to improve underrepresented recruitment (Figure 1). The first, seemingly basic solution is to have prespecified enrollment targets for certain underrepresented groups based on census and disease prevalence data.9 The FDA’s new guidance on recruitment of underrepresented populations formally recommends that enrollment goals be submitted along with investigational drug or device applications.43 Loosening eligibility criteria—such as including all New York Heart Association classes of heart failure or any multitude of natriuretic peptide levels in cardiovascular trials2,20— to allow for those with more comorbidities may additionally lead to greater representation given the higher burden of chronic diseases among minority groups. Selecting medical institutions that serve a higher prevalence of underrepresented groups for clinical trial sites can facilitate recruitment.9 In addition, hybrid or decentralized trials that utilize community sites, home visits, and remote technology can eliminate geographic and time barriers.2
Figure 1.
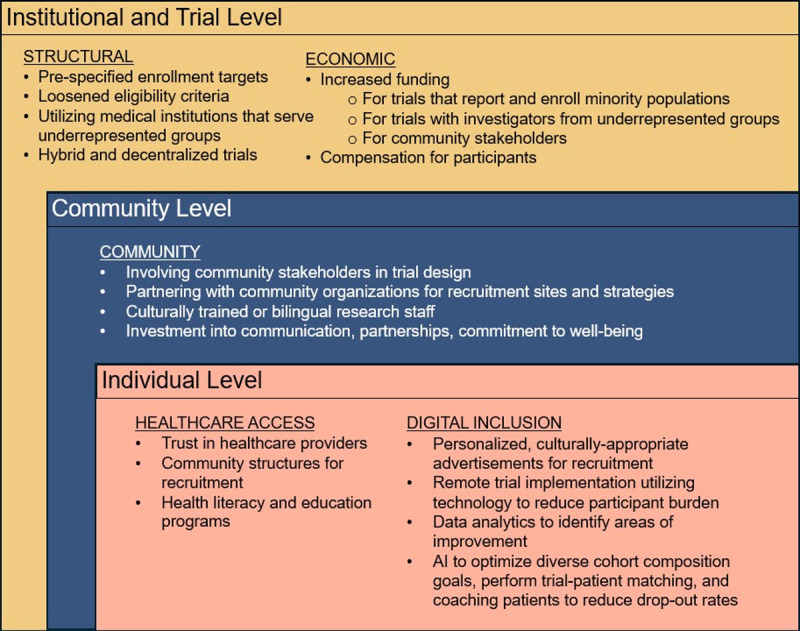
Social determinant-based toolkit for recruitment of underrepresented groups at the institutional/trial, community, and individual level. Toolkit includes structural, economic, community, healthcare access, and digital inclusion solutions.
Economic
Increased funding for trials that report and enroll representative minority populations is a clear solution to the current homogeneous state of clinical trials. The National Institutes of Health, one of the largest federal funders of clinical trials, has dozens of grants to increase trial funding for underrepresented groups, and even industry and pharmaceutical giants such as Pfizer have created specific grants for funding research that aims to reduce disparities.44,45 Increased funding for trials with investigators from underrepresented groups can also indirectly increase minority representation in clinical trials.2,9 For enrollment and retention, greater funding for community stakeholders to increase trial accessibility, and flexible funding for longer enrollment periods, recruitment materials, and more research staff can aid in increasing the diversity representation in clinical trials.9,10 Finally, significant debate surrounds compensation of trial participants given the concern for coercion and is limited by processes through the Institutional Review Board.46 However, identifying ways of compensation and assistance for transportation, time, and effort ensures fair compensation and combats undue burden on these individuals, aligning with ethical principles.46
Community
The social and community context of the trial is another determinant that can be targeted to improve representation. Involving community members in the design of the trial, such as through a community advisory board, can improve enrollment and retention by enhancing the community’s perception and appropriateness of trial materials and by refining the research question itself to maximize benefit.9,47,48 Partnering with community organizations for recruitment sites and strategies has been shown to increase enrollment, likely through cultural, linguistic, and geographic improvement in recruitment strategies and inherent trust in the community partner involved.40,49 Having culturally trained and bilingual or multilingual research staff also can improve community perceptions and improve comprehension of the study’s materials.20,50 Other principles of community engagement include clear investment into the community with good communication, sustainable partnerships, and long-term commitments to the community’s well-being.20 In fact, community investment before research needs arise is crucial in building trust and reciprocity to allow for true partnerships between investigators and the communities they seek to reach.9
Healthcare Access
Lack of access to health care, whether through geographic, financial constraints, or perceptions of the medical institution, presents a challenging barrier for enrollment of underrepresented groups in clinical trials. As previously mentioned, selecting medical institutions that serve more underrepresented groups or decentralizing trials to include community health sites or home visits can help remove physical barriers to healthcare access and trial enrollment for underrepresented groups.2,10 Primary care providers are another key mediator to trial participation, as studies have shown that relationships and satisfaction with primary care physicians increase trial enrollment and follow-through.34 Clinic-level interventions to facilitate enrollment, such as modifying clinic workflow, electronic health record alerts, a full-time onsite study coordinator, and dedicated time to participate in research are several avenues to improve enrollment through primary care providers.34 Further, using community structures and figures outside the medical field, such as churches and barbershops, as sites of recruitment and intervention has been used with success, in part by overcoming geographic barriers but also motivating health behaviors through social networks.2 Finally, trial materials and processes should have appropriate health literacy with available education programs.48
Digital Inclusion
Digital health tools can help increase the diversity of clinical trials. Trials can utilize remote technology to recruit and implement the interventions themselves, lowering participant burden. The CHIEF-HF (A Study on Impact of Canagliflozin on Health Status, Quality of Life and Functional Status in Heart Failure) trial, which evaluated the effect of sodium-glucose co-transporter 2 inhibitors on heart failure symptoms, was performed remotely with recruitment through the study website, electronic informed consent, direct home delivery of study medication, completion of the primary end point survey by mobile application, and a Fitbit to monitor activity.51 Likewise, the DeTAP (Decentralized Trial in Afib Patients) trial used mobile applications, remote blood pressure, and electrocardiographic monitoring for oral anticoagulation adherence.52 Using this technology, these trials experienced rapid recruitment and high participant engagement. These methods can be leveraged to recruit and maintain underrepresented groups by reducing healthcare access, geographic, time, and financial barriers, particularly when provided to close the digital divide.53,54 Digital care transformation programs can similarly enroll equitable numbers of underrepresented groups through health technology with a goal to improve health outcomes. Remote medication management, mobile phone applications, social media, and web-based programs are all successful avenues to broaden recruitment and trial implementation to improve health outcomes.53,54,55,56 Other opportunities in which digital technology can improve equity in trials include personalized and culturally appropriate advertisements for trial marketing, data analytics to identify drop-outs, and care coordination technologies.54 Finally, artificial intelligence can be utilized to optimize diverse cohort composition goals, perform trial-patient matching through natural language processing, and coach patients to reduce drop-out rates.57
Conclusion
In conclusion, clinical trials need to include populations that represent those with the disease under study. Historically marginalized and underrepresented communities are disproportionately affected by cardiometabolic diseases, and novel strategies are needed to address the barriers to clinical trial enrollment. Leveraging a social determinants-based approach provides a toolkit that targets these barriers using structural, economic, community, healthcare access, and technology solutions. Prespecified enrollment targets, broadening eligibility criteria, increased funding for research focused on underrepresented groups, and decentralizing trials are tangible goals for investigators to improve trial representation. Investment in communities through early partnerships between investigators and community stakeholders, addressing healthcare access and literacy, and ensuring culturally trained and bilingual or multilingual research staff can improve trial recruitment and retainment and positively impact a community. Finally, reducing participant burden by offering remote enrollment, medication management, and monitoring can facilitate recruitment, while digital care transformation programs can directly improve the health of underrepresented groups. Future research should continue to implement these tools for constructive improvement in trial design, enrollment, implementation, and outcomes.
Key Points
Historically marginalized communities are disproportionately affected by cardiometabolic diseases yet are underrepresented in clinical trials that investigate needed interventions.
Barriers to recruitment and enrollment of underrepresented groups exist at the institutional, trial, community and individual level.
Leveraging a social determinants-based approach provides a toolkit that targets these barriers using structural, economic, community, healthcare access, and technology solutions.
Acknowledgements
The authors thank Summer Ngo for her administrative work and assistance with the figure.
Funding Statement
Dr. Fatima Rodriguez reports equity from Carta Healthcare and HealthPals and is a consultant for HealthPals, Novartis, NovoNordisk, Esperion Therapeutics, Movano Health, Kento Health, Inclusive Health, Edwards, Arrowhead Pharmaceuticals, HeartFlow, iRhythm, Amgen, and Cleerly Health outside the submitted work; she receives grant funding from the NIH National Heart, Lung, and Blood Institute (1K01HL144607; R01HL168188), the American Heart Association/Harold Amos Medical Faculty Development program, and the Doris Duke Foundation (Grant #2022051).
Competing Interests
The other authors have no competing interests to declare.
References
- 1.National Institutes of Health (Internet). Bethesda, MD: National Institutes of Health; c2024. NIH’s Definition of a Clinical Trial; updated 2024. June 10 [cited 2024 Jun 15]. Available from: https://grants.nih.gov/policy/clinical-trials/definition.htm [Google Scholar]
- 2.Kelsey MD, Patrick-Lake B, Abdulai R, et al. Inclusion and diversity in clinical trials: Actionable steps to drive lasting change. Contemp Clin Trials. 2022. May;116:106740. doi: 10.1016/j.cct.2022.106740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Food and Drug Administration [Internet]. Silver Spring, MD: U.S. Food and Drug Administration; c2024. Drug Trials Snapshots Summary Report; 2023; 2024 [cited 2024 Jun 1]. Available from: https://www.fda.gov/media/178602/download?attachment [Google Scholar]
- 4.U.S. Census [Internet]. Washington, DC: U.S. Department of Commerce; c2024. Jensen E, Jones N, Rabe M, et al. 2020. U.S. population more racially and ethnically diverse than measured in 2010; updated 2023 Oct 11 [cited 2024 Jun 1]. Available from: https://www.census.gov/library/stories/2021/08/2020-united-states-population-more-racially-ethnically-diverse-than-2010.html [Google Scholar]
- 5.Buffenstein I, Kaneakua B, Taylor E, et al. Demographic recruitment bias of adults in United States randomized clinical trials by disease categories between 2008 to 2019: A systematic review and meta-analysis. Sci Rep. 2023. Jan;13:42. doi: 10.1038/s41598-022-23664-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeCormier Plosky W, Ne’eman A, Silverman BC, et al. Excluding people with disabilities from clinical research: Eligibility criteria lack clarity and justification. Health Aff (Millwood). 2022. Oct;41(10):1423-1432. doi: 10.1377/hlthaff.2022.00520 [DOI] [PubMed] [Google Scholar]
- 7.Sardar MR, Badri M, Prince CT, Seltzer J, Kowey PR. Underrepresentation of women, elderly patients, and racial minorities in the randomized trials used for cardiovascular guidelines. JAMA Intern Med. 2014. Nov;174(11):1868-70. doi: 10.1001/jamainternmed.2014.4758 [DOI] [PubMed] [Google Scholar]
- 8.Sarraju A, Valencia A, Knowles JW, Maron DJ, Rodriguez F. Diverse Racial/Ethnic Group Underreporting and Underrepresentation in High-Impact Cholesterol Treatment Trials. Circulation. 2021. Jun 15;143(24):2409-2411. doi: 10.1161/CIRCULATIONAHA.120.050034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Academies [Internet]. Washington, DC: National Academies Press; c2024. National Academies of Sciences, Engineering, and Medicine. Bibbins-Domingo K, Helman A, editors. Improving representation in clinical trials and research: building research equity for women and underrepresented groups; 2022. [cited 2024 Sep 4]. Available from: https://nap.nationalacademies.org/catalog/26479/improving-representation-in-clinical-trials-and-research-building-research-equity [PubMed] [Google Scholar]
- 10.Bodicoat DH, Routen AC, Willis A, et al. Promoting inclusion in clinical trials-a rapid review of the literature and recommendations for action. Trials. 2021. Dec 4;22(1):880. doi: 10.1186/s13063-021-05849-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehrhardt S, Appel LJ, Meinert CL. Trends in National Institutes of Health Funding for Clinical Trials Registered in ClinicalTrials.gov. JAMA. 2015. Dec 15;314(23):2566-7. doi: 10.1001/jama.2015.12206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner BE, Steinberg JR, Weeks BT, Rodriguez F, Cullen MR. Race/ethnicity reporting and representation in US clinical trials: a cohort study. Lancet Reg Health Am. 2022. Jul;11:100252. doi: 10.1016/j.lana.2022.100252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Unger JM, Hershman DL, Osarogiagbon RU, et al. Representativeness of Black Patients in Cancer Clinical Trials Sponsored by the National Cancer Institute Compared With Pharmaceutical Companies. JNCI Cancer Spectr. 2020. Apr 24;4(4):pkaa034. doi: 10.1093/jncics/pkaa034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao H, Vaidya R, Liu F, Chang X, Xia X, Unger JM. Sex, Racial, and Ethnic Representation in COVID-19 Clinical Trials: A Systematic Review and Meta-analysis. JAMA Intern Med. 2023. Jan 1;183(1):50-60. doi: 10.1001/jamainternmed.2022.5600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonald KE, Schwartz AE, Sabatello M. Eligibility criteria in NIH-funded clinical trials: Can adults with intellectual disability get in? Disabil Health J. 2022. Oct;15(4):101368. doi: 10.1016/j.dhjo.2022.101368 [DOI] [PubMed] [Google Scholar]
- 16.Kanapuru B, Fernandes LL, Baines A, et al. Eligibility criteria and enrollment of a diverse racial and ethnic population in multiple myeloma clinical trials. Blood. 2023. Jul 20;142(3):235-243. doi: 10.1182/blood.2022018657 [DOI] [PubMed] [Google Scholar]
- 17.Webb Hooper M, Asfar T, Unrod M, et al. Reasons for Exclusion from a Smoking Cessation Trial: An Analysis by Race/Ethnicity. Ethn Dis. 2019. Jan 17;29(1):23-30. doi: 10.18865/ed.29.1.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guadagnolo BA, Petereit DG, Helbig P, et al. Involving American Indians and medically underserved rural populations in cancer clinical trials. Clin Trials. 2009. Dec;6(6):610-7. doi: 10.1177/1740774509348526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riner AN, Girma S, Vudatha V, et al. Eligibility Criteria Perpetuate Disparities in Enrollment and Participation of Black Patients in Pancreatic Cancer Clinical Trials. J Clin Oncol. 2022. Jul 10;40(20):2193-2202. doi: 10.1200/JCO.21.02492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Defilippis EM, Echols M, Adamson PB, et al. Improving Enrollment of Underrepresented Racial and Ethnic Populations in Heart Failure Trials: A Call to Action From the Heart Failure Collaboratory. JAMA Cardiol. 2022. May 1;7(5):540-548. doi: 10.1001/jamacardio.2022.0161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta DK, de Lemos JA, Ayers CR, Berry JD, Wang TJ. Racial Differences in Natriuretic Peptide Levels: The Dallas Heart Study. JACC Heart Fail. 2015. Jul;3(7):513-519. doi: 10.1016/j.jchf.2015.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seidler EM, Keshaviah A, Brown C, Wood E, Granick L, Kimball AB. Geographic distribution of clinical trials may lead to inequities in access. Clin Invest. 2014;4:373-380. doi: 10.4155/cli.14.21 [DOI] [Google Scholar]
- 23.Galsky MD, Stensland KD, McBride RB, et al. Geographic accessibility to clinical trials for advanced cancer in the United States. JAMA Intern Med. 2015. Feb;175(2):293-5. doi: 10.1001/jamainternmed.2014.6300 [DOI] [PubMed] [Google Scholar]
- 24.Agency for Healthcare Research & Quality [Internet]. Rockville, MD: US Department of Health and Human Services; c2024. National Healthcare Quality & Disparities Report; reviewed 2015. Jun [cited 2024 Sep 4]. Available from: http://www.ahrq.gov/research/findings/nhqrdr/nhqdr14/key1.html [Google Scholar]
- 25.Tikkanen RS, Woolhandler S, Himmelstein DU, et al. Hospital Payer and Racial/Ethnic Mix at Private Academic Medical Centers in Boston and New York City. Int J Health Serv. 2017. Jul;47(3):460-476. doi: 10.1177/0020731416689549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Occa A, Morgan SE, Potter JE. Underrepresentation of Hispanics and Other Minorities in Clinical Trials: Recruiters’ Perspectives. J Racial Ethn Health Disparities. 2018. Apr;5(2):322-332. doi: 10.1007/s40615-017-0373-x [DOI] [PubMed] [Google Scholar]
- 27.Giuliano AR, Mokuau N, Hughes C, et al. Participation of minorities in cancer research: the influence of structural, cultural, and linguistic factors. Ann Epidemiol. 2000. Nov;10(8 Suppl):S22-34. doi: 10.1016/s1047-2797(00)00195-2 [DOI] [PubMed] [Google Scholar]
- 28.Versavel S, Subasinghe A, Johnson K, et al. Diversity, equity, and inclusion in clinical trials: A practical guide from the perspective of a trial sponsor. Contemp Clin Trials. 2023. Mar;126:107092. doi: 10.1016/j.cct.2023.107092 [DOI] [PubMed] [Google Scholar]
- 29.BiopharmaDive [Internet]. Washington, DC: BiopharmaDive; c2024. Dunn A, Pagliarulo N. Follow the money: how biopharma CEOs and workers got paid in 2018; 2019. May 28 [cited 10 June 2024]. Available from https://www.biopharmadive.com/news/biotech-pharma-ceo-employee-pay/554283/ [Google Scholar]
- 30.Getz K, Faden L. Racial disparities among clinical research investigators. Am J Ther. 2008. Jan-Feb;15(1):3-11. doi: 10.1097/MJT.0b013e31815fa75a [DOI] [PubMed] [Google Scholar]
- 31.Reza N, Tahhan AS, Mahmud N. Representation of Women Authors in International Heart Failure Guidelines and Contemporary Clinical Trials. Circ Heart Fail. 2020. Aug;13(8):e006605. doi: 10.1161/CIRCHEARTFAILURE.119.006605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yong C, Suvarna A, Harrington R, et al. Temporal Trends in Gender of Principal Investigators and Patients in Cardiovascular Clinical Trials. J Am Coll Cardiol. 2023. Jan 31;81(4):428-430. doi: 10.1016/j.jacc.2022.10.038 [DOI] [PubMed] [Google Scholar]
- 33.Clark LT, Watkins L, Piña IL, et al. Increasing Diversity in Clinical Trials: Overcoming Critical Barriers. Curr Probl Cardiol. 2019. May;44(5):148-172. doi: 10.1016/j.cpcardiol.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 34.Millar MM, Taft T, Weir CR. Clinical trial recruitment in primary care: exploratory factor analysis of a questionnaire to measure barriers and facilitators to primary care providers’ involvement. BMC Prim Care. 2022. Dec 3;23(1):311. doi: 10.1186/s12875-022-01898-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katz RV, Kegeles SS, Kressin NR, et al. Awareness of the Tuskegee Syphilis Study and the US presidential apology and their influence on minority participation in biomedical research. Am J Public Health. 2008. Jun;98(6):1137-42. doi: 10.2105/AJPH.2006.100131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wendler D, Kington R, Madans J, et al. Are racial and ethnic minorities less willing to participate in health research? PLoS Med. 2006. Feb;3(2):e19. doi: 10.1371/journal.pmed.0030019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.U.S. Department of Labor [Internet]. Washington, DC: U.S. Department of Labor; c2024. Earnings and Ratios: Women’s Bureau; 2024. [cited 2024 Sep 4]. Available from: https://www.dol.gov/agencies/wb/data/earnings [Google Scholar]
- 38.Kaiser Family Foundation [Internet]. San Francisco, CA: Kaiser Family Foundation; c2024. Drake P, Burns A. Working-age adults with disabilities living in the community; 2024. Jan 4 [cited 2024 Sep 4]. Available from: https://www.kff.org/medicaid/issue-brief/working-age-adults-with-disabilities-living-in-the-community/ [Google Scholar]
- 39.Goodman JM, Richardson DM, Dow WH. Racial and Ethnic Inequities in Paid Family and Medical Leave: United States, 2011 and 2017–2018. Am J Public Health. 2022. Jul;112(7):1050-1058. doi: 10.2105/AJPH.2022.306825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonevski B, Randell M, Paul C, et al. Reaching the hard-to-reach: a systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC Med Res Methodol. 2014. Mar 25;14:42. doi: 10.1186/1471-2288-14-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friedman SH, Cunningham CO, Lin J, Haramati LB, Levsky JM. Having a Primary Care Provider is the Strongest Predictor of Successful Follow-up of Participants in a Clinical Trial. J Am Board Fam Med. 2020. May-Jun;33(3):431-439. doi: 10.3122/jabfm.2020.03.190018 [DOI] [PubMed] [Google Scholar]
- 42.Gadegbeku CA, Stillman PK, Huffman MD, Jackson JS, Kusek JW, Jamerson KA. Factors associated with enrollment of African Americans into a clinical trial: results from the African American study of kidney disease and hypertension. Contemp Clin Trials. 2008. Nov;29(6):837-42. doi: 10.1016/j.cct.2008.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.U.S. Food & Drug Administration [Internet]. Silver Spring, MD: U.S. Food & Drug Administration; c2024. Diversity plans to improve enrollment of participants from underrepresented racial and ethnic populations in clinical trials; draft guidance for industry; 2022. Apr [cited 2024 Sep 4]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/diversity-plans-improve-enrollment-participants-underrepresented-racial-and-ethnic-populations [Google Scholar]
- 44.National Institute of Health (Internet). Bethesda, MD: National Institute of Health; c2024. Diversity related funding opportunity announcements; 2024. [cited 2024 Sep 4]. Available from: https://extramural-diversity.nih.gov/guidedata/data [Google Scholar]
- 45.Pfizer [Internet]. New York, NY: Pfizer; c2024. Covington. A report to Pfizer Inc. on its efforts to promote racial equity, diversity, and inclusion; 2024. May [cited 2024 Sep 4]. Available from: https://cdn.pfizer.com/pfizercom/Pfizer_Racial_Equity_Assessment_Report_031424.pdf [Google Scholar]
- 46.Largent EA, Fernandez Lynch H. Paying Research Participants: Regulatory Uncertainty, Conceptual Confusion, and a Path Forward. Yale J Health Policy Law Ethics. 2017. Winter;17(1):61-141. PMID: 29249912 [PMC free article] [PubMed] [Google Scholar]
- 47.Las Nueces D, Hacker K, Digirolamo A, Hicks LS. A systematic review of community-based participatory research to enhance clinical trials in racial and ethnic minority groups. Health Serv Res. 2012. Jun;47(3 Pt 2):1363-86. doi: 10.1111/j.1475-6773.2012.01386.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crocker JC, Ricci-Cabello I, Parker A, et al. Impact of patient and public involvement on enrolment and retention in clinical trials: Systematic review and meta-analysis. BMJ. 2018. Nov 28;363:k4738. doi: 10.1136/bmj.k4738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Centers for Disease Control and Prevention [Internet]. Atlanta, GA: Center for Disease Control and Prevention; c2024. Principles of Community Engagement. 2nd ed; 2011. Jun [cited 2024 Sep 4]. Available from: https://www.atsdr.cdc.gov/communityengagement/index.html [Google Scholar]
- 50.Haynes N, Kaur A, Swain J, Joseph JJ, Brewer LC. Community-Based Participatory Research to Improve Cardiovascular Health Among US Racial and Ethnic Minority Groups. Curr Epidemiol Rep. 2022;9(3):212-221. doi: 10.1007/s40471-022-00298-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spertus JA, Birmingham MC, Nassif M, et al. The SGLT2 inhibitor canagliflozin in heart failure: the CHIEF-HF remote, patient-centered randomized trial. Nat Med. 2022. Apr;28(4):809-813. doi: 10.1038/s41591-022-01703-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sarraju A, Seninger C, Parameswaran V, et al. Pandemic-proof recruitment and engagement in a fully decentralized trial in atrial fibrillation patients (DeTAP). NPJ Digit Med. 2022. Jun 28;5(1):80. doi: 10.1038/s41746-022-00622-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kasahara A, Mitchell J, Yang J, Cuomo RE, McMann TJ, Mackey TK. Digital technologies used in clinical trial recruitment and enrollment including application to trial diversity and inclusion: A systematic review. Digit Health. 2024. Mar 28;10:20552076241242390. doi: 10.1177/20552076241242390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Broadwin C, Azizi Z, Rodriguez F. Clinical Trial Technologies for Improving Equity and Inclusion in Cardiovascular Clinical Research. Cardiol Ther. 2023. Jun;12(2):215-225. doi: 10.1007/s40119-023-00311-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blood AJ, Cannon CP, Gordon WJ, et al. Results of a Remotely Delivered Hypertension and Lipid Program in More Than 10 000 Patients Across a Diverse Health Care Network. JAMA Cardiol. 2023. Jan 1;8(1):12-21. doi: 10.1001/jamacardio.2022.4018. Erratum in: JAMA Cardiol. 2023 Jan 1;8(1):100. doi: 10.1001/jamacardio.2022.4695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brewer LC, Jenkins S, Hayes SN, et al. Community-based, cluster-randomized pilot trial of a cardiovascular mHealth intervention: Rationale, design, and baseline findings of the FAITH! Trial. Am Heart J. 2022. May;247:1-14. doi: 10.1016/j.ahj.2022.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harrer S, Shah P, Antony B, Hu J. Artificial Intelligence for Clinical Trial Design. Trends Pharmacol Sci. 2019. Aug;40(8):577-591. doi: 10.1016/j.tips.2019.05.005 [DOI] [PubMed] [Google Scholar]


