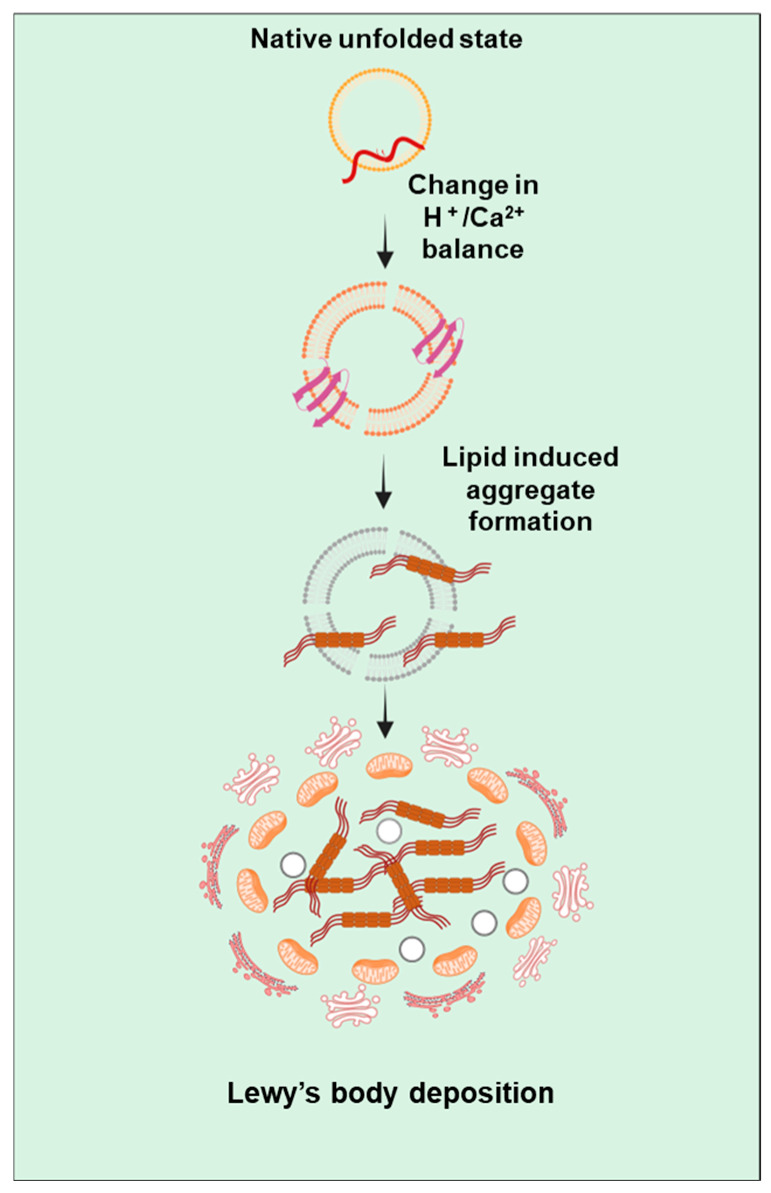
Figure 3.
Illustration depicting the pathway of α-synuclein aggregation. Under pathological conditions, α-synuclein aggregation can occur either in association with the cellular membrane or within the cytosol. When bound to the membrane, monomeric α-synuclein adopts an α-helical conformation; however, ionic dysregulation prompts a conformational shift towards membrane-bound β-sheet structures, leading to self-association and the formation of oligomers and fibrils. The haphazard accumulation of these fibrils contributes to the formation of intracytoplasmic Lewy bodies. Throughout α-synuclein fibrillogenesis, oligomers and amyloid fibrils exert significant toxicity, impairing microtubule dynamics, endoplasmic reticulum–Golgi trafficking, and mitochondrial function.