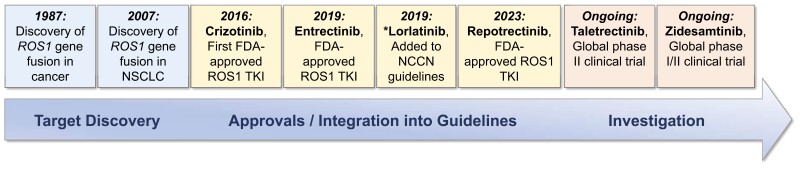
Figure 1.
Timeline of advances in ROS1 fusion-positive non–small cell lung cancer (NSCLC), including the discovery of ROS1 gene fusions, approval of ROS1 tyrosine kinase inhibitors, and development of investigational ROS1 inhibitors. Crizotinib and entrectinib are also approved by the European Medicines Agency (EMA) and globally. *Lorlatinib is not approved by the FDA for the treatment of patients with metastatic ROS1 fusion-positive NSCLC but is recommended as a subsequent treatment option by the NCCN guidelines and included in the ESMO guidelines.