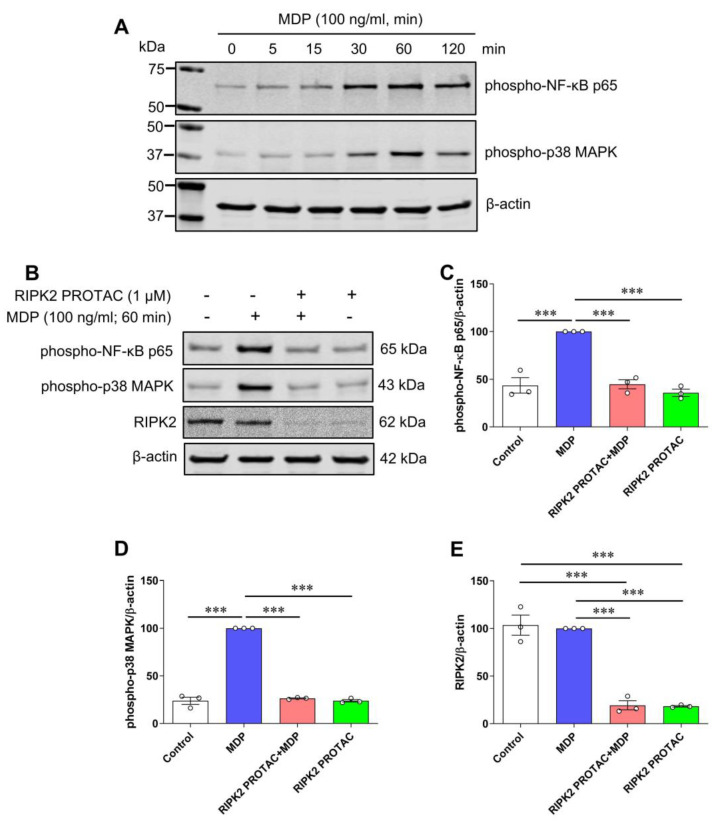
Figure 4.
RIPK2 PROTAC suppresses activation of both NF-κB p65 and MAPK p38 induced by MDP in SIM-A9 cells. (A) SIM-A9 cells were stimulated with 100 ng/mL MDP for indicated periods (0 to 120 min). Immunoblots show MDP increased phosphorylation of NF-κB p65 and MAPK p38, and 60 min incubation of MDP induced maximal effects on both phosphorylated protein levels. Data are representative of three independent experiments with similar results. (B–E) SIM-A9 cells were pretreated with 1 µM RIPK2 PROTAC for 4 h followed by 60 min incubation of 100 ng/mL MDP. After that, cells were harvested for protein extraction and Western blots. Immunoblots and graphs show that RIPK2 PROTAC completely abolished effects of MDP on phosphorylation of both NF-κB p65 (B,C) and MAPK p38 (B,D), which was associated with marked degradation of RIPK2 by its PROTAC pretreatment (B,E). One-way ANOVA with Bonferroni post-test; *** p < 0.001. Data are normalized to β-actin and represented as mean ± SEM from three independent experiments.