Fig. 2.
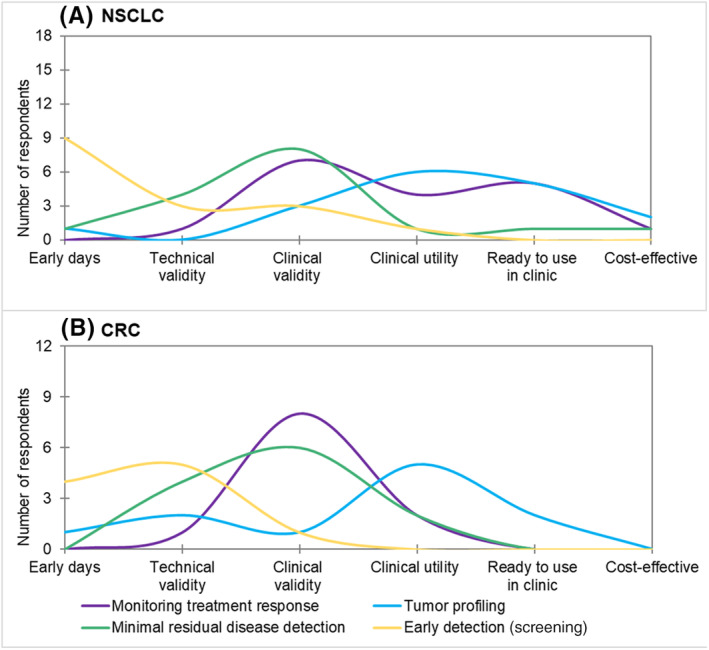
Awareness about the current stage of evidence of the different applications for ctDNA testing in the Netherlands. The y‐axis shows the total number of answers is shown, the upper limit of the y axis is the total number of respondents per tumor type. In the x‐axis shows the stages of evidence [30]. (A) Results for NSCLC. (B) Results for CRC. (1) Early days: new liquid biopsy test is developed. (2) Technical validity: ability to detect and quantify a molecular aberration. (3) Clinical validity: correlation with a clinical outcome such as prognostic value for overall survival. (4) Clinical utility: ability of the liquid biopsy to actually guide treatment decisions that improve clinical outcomes. (5) Ready to use in clinic: level of evidence where clinicians feel the test is ready for use. (6) Cost‐effective: demonstration of an economically viable test relative to the clinical benefit.
