Abstract
Introduction: Microbiota, composed of micro-organisms like bacteria, viruses, and non-pathogenic fungi, plays a crucial role in digestion, vitamin production, and protection against dangerous microbes. Several factors, including age, diet, alcohol consumption, stress, environmental microorganisms, and therapies (particularly antibiotics), as well as birth and nursing, could modify the microbiota. Recent research has highlighted its alteration and involvement in a various disease, including metabolic syndrome and acne. This systematic review aimed to identify common biomarkers and microbiota alterations shared between metabolic syndrome and acne, and to explore how the potential prebiotic activities of polyphenols may promote intestinal eubiosis. Materials and methods: A comprehensive search in PubMed and EMBASE resulted in 4142 articles, from which nine studies were selected based on specific criteria after removing duplicates and reviewing abstracts and full texts. All studies correlated the microbiota alteration in both pathologies and the activity of polyphenols in metabolic syndrome. Results: This review suggests that acne may be influenced by some of the same microorganisms involved in metabolic syndrome. While the literature highlights the effectiveness of polyphenols in treating metabolic syndrome, no studies have yet demonstrated their specific impact on acne. Conclusions: The research points to the potential benefits of polyphenols in modulating the microbiota, which could be relevant for individuals with metabolic syndrome. However, due to the limited data available, it was not possible to establish a direct correlation between metabolic syndrome and acne.
Keywords: nutraceuticals, microbiota, bacteria, metabolism, systematic review
1. Introduction
1.1. Gut Microbiota
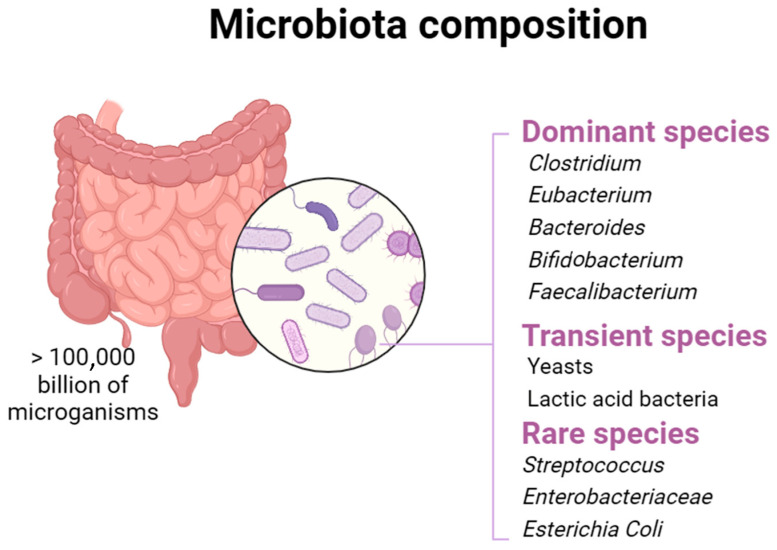
The gut microbiota, also known as the gut flora, is a community of microorganisms that reside in the human gastrointestinal tract. This community consist of a large number of cells, estimated at up to 1013, that belong to all three domains of life (Bacteria, Archaea, and Eukaryotes) [1]. The gut can host thousands of bacterial species belonging to different phyla, including Bacteroidetes, Firmicutes, Actinobacteria, Proteobacteria, Verrucomicrobia, Fusobacteria, Tenericutes, Spirochaetes, Cyanobacteria, and Saccharibacteria [2,3]. In contrast, the diversity of fungi in the human gut is limited. From samples of 98 human adults, a pyrosequencing study identified about 66 fungal genera, with Saccharomyces, Candida, and Cladosporium being the most prevalent [1,4]. The gut microbiota plays a pivotal role in maintaining host health, influencing various physiological functions and contributing to digestion, nutrient absorption, immune system maturation, and protection against harmful pathogens [5]. A healthy balance of the gut microbiota is often associated with good health, while an imbalance, known as “dysbiosis”, can be linked to various pathological conditions [6]. The gut microbiota plays a crucial role in the fermentation of non-digestible substrates such as dietary fiber and endogenous intestinal mucosa. This activity favors the development of specialized microorganisms that generate short-chain fatty acids (SCFAs) such as acetate, propionate, and butyric acid, and gas [7]. Butyric acid, which represents the main energy source for colon cells, positively impacts human health. This substance can stimulate the intestine’s production of glucose and encourage the colon’s natural death of cancer cells, which helps control blood sugar levels and preserve a healthy energy balance [8]. In addition, butyrate is essential in facilitating the consumption of high oxygen amounts by epithelial cells through β-oxidation. This activity generates a state of hypoxia, which helps to preserve the oxygen intestinal balance and prevent any imbalances in the microbiota. Propionate is transported to the liver, regulating gluconeogenesis and satiety through interaction with intestinal fatty acid receptors [9]. Acetate, the most present among SCFAs, represents an essential metabolite for bacteria proliferation, spreading into peripheral tissues and participating in cholesterol metabolism and lipogenesis [10]. Foods contribute significantly to the intestinal microbiota composition, suggesting that nutrition can prevent or alleviate diseases related to an altered microbial composition. As previously mentioned, the intestinal microbiota is involved in the food substrates fermentation, promoting or inhibiting the growth of specific types of bacteria [11]. Beyond nutrition, probiotics currently represent the most effective and safe method to specifically modify the intestinal microbiota and promote the improvement of the host’s health (Figure 1).
Figure 1.
Different microbial species inhabit the host’s gut microbiota.
1.2. Intestinal Microbiome in Adults with Acne Vulgaris
In the 1930s, Stokes and Pillsbury used experimental data to establish a connection between inflammation of the skin and microbial flora. They noted that up to 40% of people with acne showed hypochlorhydria, suggesting that hydrochloric acid deficiency could induce migration of bacteria from the colon tract to the small intestine, disturbing the balance of normal intestinal flora [12]. In recent years, it has been confirmed that hypochlorhydria represents a significant risk factor for bacterial overgrowth in the small intestine (SIBO), which can cause an increase in intestinal permeability (“leaky gut”), resulting in the development of systemic inflammation [13,14].
Bifidobacteria and Lactobacilli, normally present in the intestine as lactic-acid-producing bacteria, could play a beneficial role in the treatment of inflammatory skin diseases, including acne [15].
The gut microbiome plays a key role in the management of both systemic and local inflammation through interaction with the immune system. Microbial communities support the integrity of the intestinal barrier by converting complex, indigestible polysaccharides into short-chain fatty acids and vitamins, especially K and B12. The integrity of the intestinal barrier, supported by mucus, immune cells, IgA, and antimicrobial peptides (AMPs) produced by epithelial cells, is fundamental in preventing the access of intestinal bacteria to the bloodstream, thus contributing to the maintenance of skin homeostasis [1].
A study highlighted a significant decrease in the presence of Actinobacteria, Bifidobacterium, Butyricicoccus, Coprobacillus, and some Lactobacillus species in people suffering from acne vulgaris, accompanied by a significant increase in Proteobacteria [16,17].
Furthermore, studies have observed that acne patients have a low presence of Firmicutes and an increase of Bacteroidetes. According to one hypothesis, the stimulation of sterol regulatory element-binding protein-1 (SREBP-1), fatty acids, and sebum triglycerides occurs following an interruption of the nutritional signal, favoring the proliferation of Propionibacterium acnes [17]. Several metabolic pathways are involved in the onset of acne vulgaris, such as the mTOR pathway which is activated by high blood sugar levels [18]. This event contributes to increasing insulin-like growth factor signaling, improving the cytoplasmic expression of FoxO1, ultimately leading to the development of acne [19].
1.3. Intestinal Microbiota in Adults with Metabolic Syndrome
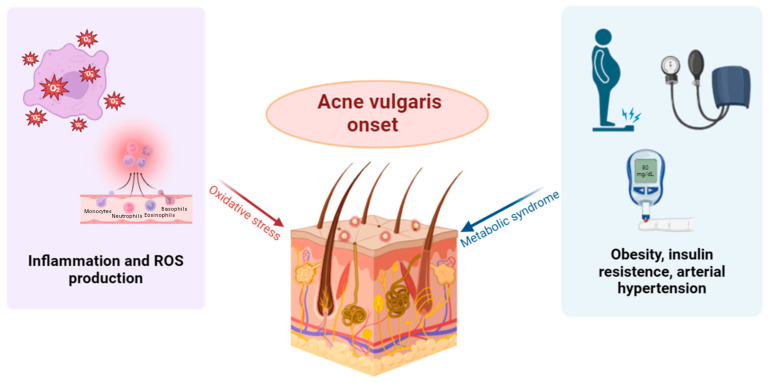
In recent years, several studies have suggested that gut microbiota dysbiosis is closely associated with many metabolic diseases, including obesity, diabetes, and non-alcoholic fatty liver disease (NAFLD). A reduction in the abundance of Akkermansia muciniphila, Faecalibacterium prausnitzii, and Bacteroides is observed, contrasted with a significant increase in the Phylum Firmicutes [20]. A metagenome analysis of a cohort of lean and obese Chinese adolescents showed a significant decrease in Bacteroides thetaiotaomicron, with a negative correlation in serum glutamate concentration [21]. Animal studies confirmed that the administration of B. thetaiotaomicron reduced serum concentrations in branched-chain amino acids and mitigated diet-induced weight gain and obesity in mice [22,23]. The gut microbiota can ferment indigestible carbohydrates, producing important metabolites such as SCFAs and succinate. These metabolites play a significant role in obesity and its comorbidities. SCFAs regulate energy balance and prevent obesity by suppressing appetite and increasing energy expenditure [24]. Several studies found significant differences in the composition of the gut microbiota between patients with type 1 diabetes (T1DM) and healthy people [25]. A study performed on Swedish participants highlighted a significant alteration of the intestinal microbiota in patients with type 2 diabetes (T2DM) [26], compared to healthy people. In fact, in the intestines of T2DM patients, a decrease in the amount of Bifidobacteria and Akkermansia and an increase in Dallella were observed [27]. Hypertension and atherosclerosis contribute to the cardiovascular risk associated with metabolic syndrome. In people with hypertension, a significant decrease in the richness, diversity, and uniformity of the intestinal microbiota is observed [28], together with a crucial increase in the Firmicutes/Bacteroidetes ratio. Other research has indicated a possible connection between intestinal microbiota (GM) and cardiovascular diseases (CVD), underlining bacterial translocation from the intestine to the heart [6,29]. The presence of live bacteria and bacterial DNA detected in atherosclerotic plaques suggests the implication of the microbiome in the development and progression of atherosclerosis. In atherosclerotic patients, the abundance of Enterobacteriaceae and Enterobacter aerogenes was significantly higher than in control samples, with an inhibitory effect on the growth of beneficial bacteria [30]. At the same time, several studies, both on animal and human models, have highlighted that intestinal dysbiosis associated with atherosclerosis could increase intestinal permeability, thus favoring the absorption of lipopolysaccharides in circulation [31]. Pathophysiological mechanisms associated with metabolic syndrome, intestinal dysbiosis, and acne vulgaris include chronic inflammation and elevated levels of oxidative stress, which may mediate the connection between the two disorders [32] (Figure 2). These data underline a close correlation between metabolic syndrome and intestinal microbiota, suggesting that the microbiota equilibrium could represent a promising therapeutic strategy to manage this condition.
Figure 2.
Oxidative stress and metabolic syndrome contribute to the development of acne vulgaris.
1.4. The Role of Polyphenols in Gut Microbiota Modulation
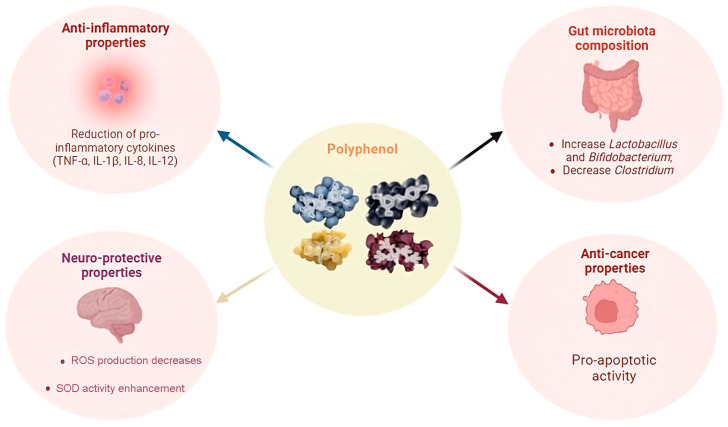
Polyphenols are natural phytochemical compounds present in many plants, fruits, and herbal remedies, including green tea, black tea, apples, coffee, bergamot, and grapes. To date, approximately 8000 different types of polyphenols have been identified [33]. Based on their chemical structure, polyphenols can be roughly divided into several categories, including condensed tannins, hydrolyzable tannins, phlorotannins, polyphenolic caffeoyl ester derivatives, flavones and flavonoids, and other substances. These polyphenolic compounds perform various beneficial activities, including antioxidant action [28,34], anti-inflammatory properties [35], and antibacterial activity [36]. Polyphenols show remarkable potential in improving several health conditions, including cancer, Alzheimer’s disease [37], diabetes, NAFLD, insulin resistance, inflammatory bowel disease, and arthritis [38,39,40,41,42,43,44]. Polyphenols offer different opportunities for interaction with the intestinal microbiota, mainly due to their wide presence in foods, herbal medicines, and plants, and thus in our diet [43,45,46]. Polyphenols are characterized by an important range of effects, thanks to different molecular structures which create multiple possible interaction with the intestinal microbiota. The low bioavailability of many monomeric polyphenols, as demonstrated by Wan and colleagues in 2021, means that a significant proportion of polyphenol can reach the gastrointestinal tract without being fully absorbed. Finally, they are used directly by microorganisms [47]. The interaction between intestinal microbiota and polyphenols occurs mainly in three distinct ways:
(1) Polyphenols influence the composition of the intestinal microbiota; (2) they alter the metabolic processes within the microbial system; (3) the intestinal microbiota itself is involved in the processing of polyphenols, influencing their structure and activity. This complex interaction plays a critical role in modulating the biological effect of polyphenols within the intestinal environment, offering greater insight into how these compounds influence host health [33].
Polyphenols are present in different sources such as Smilax china L. extracts, along with tea polyphenols and those of Aronia melanocarpa (Michx.). They have demonstrated the ability to positively influence the diversity of microbial species in the intestine, known as α-diversity. This influence has been observed in contexts such as metabolic disorders caused by a high-fat diet (HFD). Other polyphenols, including litchi pulp phenols, kaempferol, and resveratrol, have been associated with improving the α-diversity of the gut microbiota, contributing to the relief of colitis. Subsequently, polyphenols were detected as agents capable of altering the overall structure of the intestinal microbiota, evidenced by variations in β-diversity. These structural changes have been observed in individuals suffering from various pathologies, including obesity, hepatic steatosis, atherosclerosis, and colitis. Data analysis revealed a difference in the structure of the gut microbiota between sick and healthy people. Such structural changes have also been found in individuals treated with polyphenols. In conclusion, the action of polyphenols in modulating both α-diversity and β-diversity highlights their significant role in promoting intestinal health and contributing to the treatment of microbiota-related disorders [33]. A study conducted by Lu et al. in 2016 highlighted that polyphenols deriving from green tea have an important role in acne management [48]. A significant decrease in inflammatory lesions was noted on specific areas of the adults’ skin undergoing treatment, thanks to the anti-inflammatory and antioxidant action of these compounds (Figure 3).
Figure 3.
Properties of polyphenols.
2. Objectives
This systematic review aims to identify common biomarkers and microbiota alterations that may indicate significant links between metabolic syndrome and acne. Additionally, it explores how the prebiotic properties of polyphenols could contribute to maintaining intestinal eubiosis, providing a strong scientific rationale for their potential use in therapeutic and preventive approaches.
3. Materials and Methods
3.1. Search Strategy
The systematic review integrates data from experimental studies regarding the changes in the human intestinal microbiome in adults affected by acne and/or metabolic syndrome, exploring the polyphenols’ effect in improving these conditions. The keywords used to search for the articles were: “(acne) AND (gut microbiota)”, “(polyphenols) AND (acne)”, “(Lactobacillus) AND (acne) AND (Proteobacteria)”, “(polyphenols) AND (microbiome) AND (metabolic syndrome)”, “(phenols) AND (gut microbiome)”, “(gut microbiota) AND (metabolic syndrome)”.
3.2. Information Sources
The studies included in the review were retrieved from the PubMed and EMBASE databases, following the PRISMA 2020 Checklist (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement and the PICO (Patient, Intervention, Comparison, Outcome) framework [49]. All articles written in English were evaluated, and the search did not specify a specific time frame. An evaluation of references of all retrieved articles was also conducted to identify additional relevant studies not included in the aforementioned databases.
3.3. Eligibility Criteria
Studies of the following types were included in the analysis:
studies in women and men with acne;
studies in women and men with metabolic syndrome or diseases associated with this condition;
studies reporting oral polyphenol treatments.
Studies were excluded from the analysis for the following reasons:
use of topical treatments.
studies conducted on animals;
studies conducted in vitro.
3.4. Study Results
Two separate qualitative analyses were conducted to account for the heterogeneity of the results. In the first systematic analysis, the aim was to identify the composition variations of the gut microbiota in adults with acne, comparing different clinical studies conducted on men and women. The second systematic analysis compared different human experimental models to better understand variations in gut microbiota composition in people with metabolic syndrome or related conditions. This second analysis examined the effect of oral polyphenol-based treatment on the gut microbiota and showed a qualitative improvement.
3.5. Statistical Analysis
Due to the bacteria and data heterogeneity and the limited number of specific studies on the chosen topic, it was necessary to analyze the data at a qualitative level.
4. Results
4.1. Study Selection
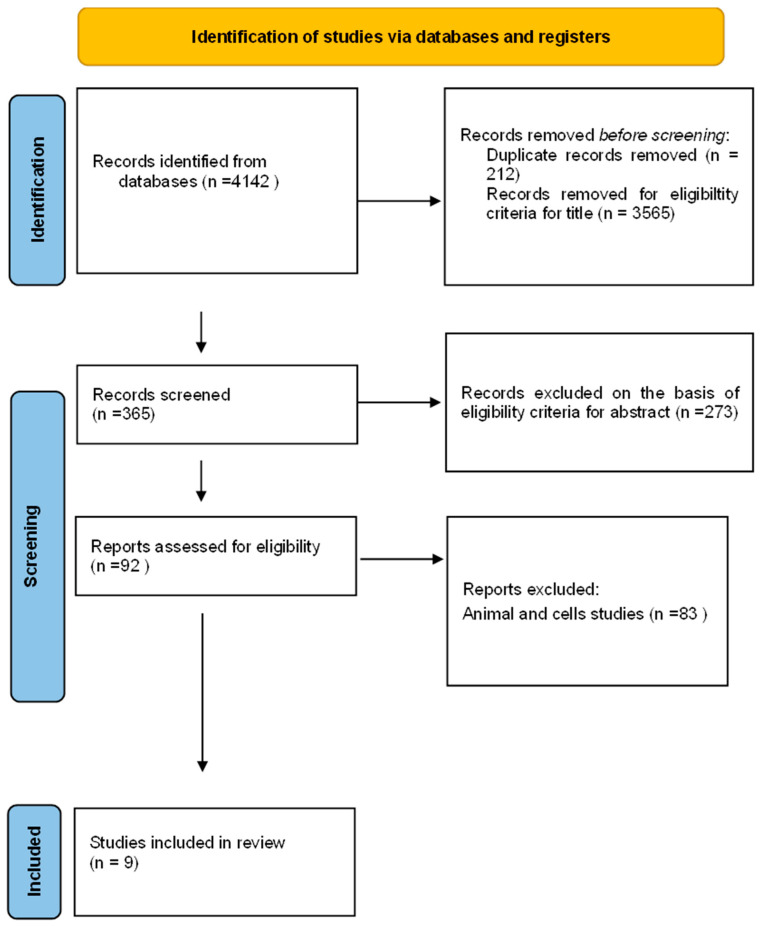
The systematic review identified 4142 documents from the literature search. Of these, 212 articles were excluded as duplicates, and 3565 were excluded as their content did not match the review’s objectives. After screening the abstract and full text, 365 articles were assessed for their eligibility, and 88 were excluded because they were reviews or animal studies, involved topical administrations, or had incomplete data. Finally, nine articles describing studies on humans were included in the qualitative analysis (systematic review) and divided into two analyses, one relating to in vivo studies on patients with acne and one relating to in vivo studies on patients with metabolic syndrome. The literature search process and the selection of articles are illustrated in the following Figure (PRISMA flowchart Figure 4).
Figure 4.
PRISMA flowchart.
4.2. Study Characteristics
4.2.1. Systematic Review on Gut Microbiome Changes in Acne Patients
Table 1 summarizes the main features of the four articles describing the differences in gut microbiome composition between healthy and acne patients. Formerly, it has been observed that gut microbiota plays a crucial role in the pathogenesis of acne. Hui-Min Yan et al. (2018) conducted a study to identify the differences in gut microbiota between acne patients and healthy individuals [28]. The study enrolled 31 healthy individuals (free from any skin disease) and 31 individuals with moderate to severe acne vulgaris. The distribution of the phyla Proteobacteria and Actinobacteria, the two major populations in the human gut, shows a significant disparity between acne patients and controls [28]. The phylum Proteobacteria is more abundant in acne patients, including many pathogenic bacteria such as E. coli, Salmonella, and Vibrio cholerae [28]. In addition, a decreased level of the genera Bifidobacterium, Butyricicoccus, Coprobacillus, Lactobacillus, and Allobaculum is observed in acne patients [28]. Bifidobacterium are Gram-positive bacilli belonging to the phylum Actinobacteria, while Lactobacillus are Gram-positive bacilli belonging to the phylum Firmicutes. A subsequent analysis conducted by Katherine G. Thompson et al. (2020) was published based on a case-control study investigating the skin and gut microbiota in eight acne patients before and after oral minocycline administration, comparing them with eight controls [50]. Oral antibiotics are the “gold standard” for the treatment of moderate to severe acne. So far, it is not known whether the efficacy of oral antibiotics in acne treatment is due to systemic absorption and, therefore, to the direct action of the antibiotic on the acne lesion, or an indirect action, due to the action of the antibiotic on the gut microbiome. Compared to controls, acne patients showed a reduced gut ratio of Firmicutes to Bacteroidetes. Following oral antibiotic treatment, acne patients’ microbiome underwent a statistically significant increase in Bacteroidetes levels. Furthermore, the gut microbiota in acne patients at baseline, compared to controls, was observed to be poor in Gram-positive probiotic bacilli such as Lactobacillus iners, Lactobacillus zeae, and Bifidobacterium animalis [50]. It has also been observed that increased intestinal permeability may allow the passage of lipopolysaccharides (LPS) by Gram-negative gut bacteria into systemic circulation, promoting inflammation and the development of acne lesions through their action on toll-like receptors TLR2 and TLR4, which are overexpressed in inflammatory acne [50]. Deng and colleagues studied whether the gut microbiota is altered in individuals with acne. It was shown that differences in microbial diversity were found between patients with acne and controls. At the phylum level, the Firmicutes abundance was lower in the patient group compared to healthy controls, while that of Bacteroidetes was higher [51]. The study conducted by Manzhalii et al. examined the effect of Escherichia coli Nissle on intestinal inflammation and the intestinal microbiota in patients with intestinal dermatoses. After treatment with E. coli Nissle, a significant reduction in pathogenic flora and an increase in Bifidobacteria and Lactobacilli were observed in the feces of treated patients. This change in the microbiota was associated with a clinical improvement, with normalization of stool consistency, color, and odor. Additionally, an increase in serum IgA levels and suppression of proinflammatory cytokines were found, indicating a beneficial effect of probiotic therapy with E. coli Nissle in intestinal dermatoses [52].
Table 1.
Studies highlighting changes in the gut microbiota in adults affected by acne.
| Study/Year | Population | Age | Sex | Bacteria Phylum |
Results |
|---|---|---|---|---|---|
| Hui-Min Yan et al., 2019 [28] |
31 healthy adults; 31 adults with acne | Healthy adults: 22.87 ± 3.65 Acne: 22.16 ± 4.24 |
Male 8 Female 26 |
Actinobacteria
Proteobacteria |
It has been demonstrated that the phylum Actinobacteria is lower in patients with acne compared to the control group, while Proteobacteria is higher compared to the control group. |
| Yongqiong Deng et al., 2018 [51] |
43 healthy adults; 43 adults with acne | Healthy adults: 20.24 ± 3.06 Acne: 20.19 ± 2.59 |
Male 17 Female 26 |
Bacteroidetes
Firmicutes |
In the study, differences in microbial diversity were found for patients with acne and controls. At the phylum level, the Firmicutes abundance was lower in the patient group compared to healthy controls, but that of Bacteroidetes was higher. |
| Katherine G. Thompson et al., 2020 [50] |
8 healthy adults; 8 adults with acne | Healthy adults: 23~34. Acne: 20~32 |
NA |
Firmicutes
Bacteroidetes |
It has been observed that at the phylum level, the abundance of Firmicutes was lower in the patient group compared to healthy controls, but that of Bacteroidetes was higher. |
| Elina Manzhalii et al., 2016 [52] |
20 healthy adults; 37 adults with acne |
Healthy adults: 28 ± 2.5 Acne: 29 ± 3.1 |
Actinobacteri (Genus Bifidobcteria) Firmicutres (Genus Lactobacteria) | In treatment with E. coli Nissle, a significant reduction in pathogenic flora and an increase in Bifidobacteria and Lactobacilli were observed in the feces of treated patients. |
4.2.2. Systematic Review on Changes in the Gut Microbiome in Adults with Metabolic Syndrome or Associated Pathological Conditions
Table 2 presents an overview of the articles considered in the systematic review, which aim to describe the changes in the gut microbiome in adults with metabolic syndrome or pathological conditions closely related to it. In addition, the improvements in the microbiome following the administration of substances/supplements/diets/drinks with a high polyphenolic content are described. In the study carried out by Moreno-Indias et al. in 2016, the possible prebiotic effect deriving from moderate consumption of red wine polyphenols on the modulation of the composition of the gut microbiota and the improvement of risk factors associated with metabolic syndrome in obese patients was evaluated [53]. In the study, 20 patients were involved, 10 of whom were healthy adults. An increased level of Proteobacteria and Firmicutes has been demonstrated in patients with metabolic syndrome compared to healthy adults. However, after the red wine and de-alcoholized red wine intake, no significant differences at the phylum level emerged between the two study groups [53]. Adults with metabolic syndrome, after the periods of treatment based on red wine polyphenols, presented a higher concentration of Fusobacteria and Bacteroidetes and a significant decrease in Firmicutes, compared to baseline [54]. Within Firmicutes, in patients with metabolic syndrome, a lower number of bacteria from the Clostridium group and the Clostridium histolyticum group was found, accompanied by a significant increase in bacteria belonging to the Blautia coccoides–Eubacterium rectale, Faecalibacterium prausnitzii, Roseburia, and Lactobacillus groups, after periods of red wine and de-alcoholized red wine intake compared to baseline. Finally, it was observed that the increase in Actinobacteria and Lactobacillus, together with the decrease in Clostridium histolyticum and E. coli, predicted the triglycerides’ reduction. Instead, the decrease in plasma LPS levels was associated with the Bifidobacterium growth and the reduction in Enterobacter cloacae number [53]. The study conducted by Vaiserman et al. in 2017 on the Ukrainian population demonstrates the changes in the composition of the gut microbiota between a group of “normal weight” individuals and a group of patients classified as “obese” based on body mass index (BMI). The study found a significant increase in Firmicutes and a higher Firmicutes/Bacteroidetes ratio in overweight and obese people compared to normal-weight adults in the Ukrainian population. The results obtained in the Ukrainian population are consistent with those obtained in other populations [54].
Table 2.
Studies highlighting changes in the gut microbiome in adults with metabolic syndrome or related conditions.
| Study/Year | Population | Age | Sex | Bacteria Phylum |
Polyphemols | Results |
|---|---|---|---|---|---|---|
| Moreno-Indias et al., 2016 [53] |
10 healthy adults; 10 adults with metabolic syndrome |
48 ± 2 years (range 45–50 years) | NA |
Fusobacteriota
Bacteroidetes Firmicudes |
Red wine polyphenols | Significant increase in Fusobacteria and Bacteroidetes, and significant decrease in the Clostridium and Firmicudes group, in patients with metabolic syndrome, compared to baseline. |
| Vaiserman et al., 2017 [54] |
34 adults with BMI ≤18.5–24; 11 adults with BMI >30 |
Mean age 44.2 years | Adults with BMI ≤18.5–24: 9 male and 25 female; adults with BMI >30: 4 male and 7 female |
Actinobacteria
Firmicutes Bacteroidetes |
NA | Adults with a high BMI have a higher level of Firmicutes and a lower level of Bacteroidetes than adults with normal weight. |
| Ni et al., 2018 [55] |
11 healthy adults; 12 adults with metabolic syndrome | 60–89 years | Heathy people: 6 male and 5 female; metabolic syndrome: 8 male and 4 female |
Firmicutes
Bacteroidetes Actinobacteria Proteobacteria |
Yangyin Tiluo Decoction (YTD) | Adults treated with the Chinese herbal formula (Yangyin Tiluo Decoction) were able to reduce the abundance of potentially pathogenic bacteria and lipoprotein A. |
| Meir et al., 2021 [56] |
294 adults with NAFLD adulted to a different diet:
|
51 years | 260 male and 34 female | Firmicutes | A polyphenol-rich diet (440–800 mg/day) | Adults treated with a diet rich in polyphenols (green MED) demonstrated a reduction in non-alcoholic hepatic steatosis, beneficial changes in cardiometabolic and inflammatory parameters, and improvements in intestinal bacterial flora. |
| Zhang et al., 2022 [57] |
10 healthy adults; 26 with pre-diabetes |
35 ± 2 years | Heathy adults: 3 male and 7 female; pre-diabetes adults: 14 male and 12 female |
Actinobacteria
Firmicutes |
Red berry polyphenols | Supplementation with red fruit polyphenols (RRB) reduced total cholesterol and LDL levels in adults with pre-diabetes; RRB increased Eubacterium eligens and reduced Ruminococcus gnavus, while RRB + FOS increased Bifidobacterium spp. and reduced Blautia wexlerae compared to baseline. |
The traditional Chinese herbal formula Yangyin Tiluo Decoction (YTD), rich in polyphenols and used in the study by Ni et al. in 2017, mitigates metabolic syndrome and cardiovascular diseases [55]. In this study, the gut microbiota alterations in elderly people with metabolic syndrome, following natural treatment based on polyphenols, were investigated. In these patients, YTD can improve intestinal microbial composition and reduce the metabolic markers of cerebrovascular disease [55]. Older adults with metabolic syndrome showed an increase in Lactobacillus and Bifidobacterium species, accompanied by a reduction in butyrate-producing bacteria compared to healthy controls. Although Lactobacillus and Bifidobacterium are generally considered beneficial bacteria and often used as probiotics, studies have shown an increase in interleukin 1β (IL-1β) concentrations and a potential increase in the inflammatory response following their presence. YTD can reduce the lipoprotein A concentration, a known risk factor for cardiovascular disease and atherosclerosis. The decrease in lipoprotein A reflects the clinical efficacy of YTD in mitigating metabolic syndrome in older individuals [55].
A correct diet is essential to maintain the balance of the intestinal bacterial flora. According to Meir et al. (2021), a “green” diet rich in vegetable-derived polyphenols brings benefits to individuals affected by hepatic steatosis, abdominal obesity, and dyslipidemia [56]. The hypothesis has been advanced that the intestinal microbiota plays a crucial role in the pathogenesis of NAFLD, as dysbiosis appears to be linked to the modulation of fat and lipid hepatic metabolism. In this study, nine bacteria at the genus level were initially identified as significantly related to IHF (intrahepatic fat); these represented 5% of bacteria at the genus level, including Fournierella, Anaerosporobacter, Lachnospiraceae_UCG-003, and several genera of the Ruminococcaceae family [56]. Out of the nine bacteria identified, eight of them showed an association with the variation in IHF 18 months from the beginning of the study, suggesting that the presence of these bacteria is linked to changes in intrahepatic fat over time. Therefore, adopting a “green” diet enriched in polyphenols combined with physical exercise has been shown to significantly reduce the prevalence of NAFLD and improve intestinal bacterial flora [56]. Finally, in a randomized crossover clinical trial performed by Zhang et al., 2022, it is evaluated whether supplementation with polyphenol-rich red fruits (RRB), alone or in association with a prebiotic (FOS), improves cardiovascular risk biomarkers in adults with prediabetes and insulin resistance, and whether these effects are related to the modulation of the intestinal microbiota [57]. Supplementation with RRB has been shown to reduce hepatic insulin resistance, total cholesterol (TC), and LDL cholesterol (LDL-C) levels in the group of individuals with prediabetes and insulin resistance [57]. Supplementation with RRB increased the presence of E. eligens and reduced that of R. gnavus, while supplementation with RRB + FOS increased the Bifidobacterium spp. presence and decreased that of B. wexlerae. Variations in the abundances of R. gnavus, E. eligens, and B. catenulatum were significantly correlated with a reduction in hepatic insulin resistance and a decrease in TC and LDL-C [57]. R. gnavus is a fundamental component of the gut microbiota, characterized by the ability to degrade mucus [58]. Evidence suggests an association of R. gnavus with several pathologies, including diverticular disease, respiratory allergies in children, and septic arthritis. Furthermore, it is significantly more present in patients with Crohn’s disease, indicating inflammatory dysbiosis of the intestine [59]. The presence of 300% of R. gnavus was observed in the PreDM-IR group compared to the control group, which was decreased following supplementation with RRB, a result like that observed in the previously cited study on the Mediterranean “green” diet [60]. Overall, RRB supplementation improved hepatic insulin resistance, total cholesterol, and LDL cholesterol levels, with these effects being correlated with decreased R. gnavus and increased E. eligens [57]. The addition of FOS to the RRB regimen further enhanced β-cell function and increased the presence of Bifidobacterium spp., a gut bacterium known for its beneficial effect [57].
5. Discussion
Acne, in addition to being a common skin condition, plays an indicative role in detecting alterations in the gut microbiome, similar to what occurs in metabolic syndrome.
The intestinal microbiome plays a fundamental role in maintaining the host’s health, exerting a significant impact on several physiological processes, including digestion, nutrient absorption, maturation of the immune system, and defense against harmful pathogens.
An optimal balance of the microbiota is closely related to an optimal state of health, while an imbalance, known as “dysbiosis”, can be associated with various pathological conditions and diseases; systemic and/or local inflammation is a consequence of SIBO which leads to an increase in intestinal permeability and the passage of bacteria into the systemic circulation [14].
Bacterial dysbiosis in adults with acne mainly concerns an increased ratio of Firmicutes/Bacteroidetes and Proteobacteria; one notable study reported the presence of Actnobacteria, Bifidobacterium, Butyricicoccus, Coprobacillus, and some species of Lactobacillus, positive bacteria for the balance of the intestinal bacterial flora [16].
Literature studies show a specific imbalance in gut flora composition in people with metabolic syndrome. This alteration is characterized by an increase in the Firmicutes/Bacteroidetes ratio and in Bifidobacteria reduction; similar conditions can be found in individuals suffering from acne [61,62].
In this systematic review, although it was not possible to obtain a quantitative relevance of intestinal bacteria in adults with acne due to the limited amount of data available, an appreciable qualitative difference emerged. In patients suffering from acne compared to the control group, a significant increase in the presence of bacteria belonging to the Phylum Proteobacteria including pathogenic bacteria such as E. coli, Salmonella, and Vibrio cholera and an increased Firmicutes/Bacteroidetes ratio have been observed. On the other hand, lower levels of the genera Bifidobacterium (Phylum Actinobacteria), Butyricicoccus, Coprobacillus, Lactobacillus (Phylum Firmicutes), and Allobaculum are found in acne patients. It has been highlighted that, at the basal level, the microbiota of acne patients, when compared to controls, showed a significant deficiency of Gram-positive probiotic bacilli such as Lactobacillus iners, Lactobacillus zeae, and Bifidobacterium animalis [63].
Systematic analysis of the bacteria in the gut flora of people with metabolic syndrome shows similarities to changes in the microbiome of people with acne. The microbiomes of adults with metabolic syndrome or closely related pathologies have higher levels of Proteobacteria and Firmicutes than healthy controls. The Firmicutes/Bacteroides ratio is also found to be increased in people with obesity.
Treatment with polyphenols leads to an improvement in the balance of the intestinal bacterial flora, which correlates with a significant improvement in several cardiometabolic parameters [56]. These improvements include reductions in total cholesterol, triglycerides, and LDL, as well as a significant incidence of hepatic steatosis.
The bacterial flora restoration induced by polyphenols is characterized by a greater concentration of Fusobacteria and Bacteroidetes, accompanied by a notable reduction of the Firmicutes compared to the controls. Within the Firmicutes phylum, a reduced number of bacteria belonging to the Clostridium and Clostridium histolyticum groups was found, while a significant increase in bacteria was observed in the Blautia coccoides–Eubacterium rectale, Faecalibacterium prausnitzii, Roseburia, and Lactobacillus groups. In addition, the polyphenol treatment stimulated the increase of Bifidobacterium spp., an intestinal bacterium known for its beneficial effects. This review suggests that acne is caused by some of the same microorganisms that alter in metabolic syndrome. The literature only demonstrates the effectiveness of polyphenols in metabolic syndrome, with no study reporting this effect in acne. This research suggests that the potential benefits of polyphenols at the microbiota level may be relevant for patients suffering from metabolic syndrome. However, due to a lack of data, a direct correlation between metabolic syndrome and acne cannot be established.
Limitations of the Study
Some data observed could influence the results regarding the efficacy of polyphenols in metabolic syndrome or acne. Natural compounds come from different families, increasing heterogeneity. A further critical issue is represented by the bacteria heterogeneity, which prevented conducting the meta-analysis. The limited quantity of items represents a further difficulty. Current information on the potential positive effect of polyphenols on acne is limited; however, it is known that they exert a beneficial effect on the intestinal microbiota. The positive role of polyphenols in reducing cardiovascular risk also emerges from the qualitative analysis carried out on metabolic syndrome. Another important limitation is that anthropometric parameters, such as age, medication use, and the consumption of antibiotics, probiotics, and prebiotics, which can significantly influence the composition of the gut microbiota, were not taken into account.
6. Conclusions
The role of the intestinal microbiome in the development of diseases is unequivocal, even though the results currently available are limited. The gut microbiota acts decisively in the pathological processes that lead to various chronic conditions. In this context, polyphenols derived from plant species seems to hold great potential in the management and prevention of disease. The positive interaction between polyphenols and microbiota opens the way to new strategies for the therapeutic management of chronic diseases and the development of personalized therapies aimed at improving patient recovery during rehabilitation. It is therefore necessary to highlight how implementing a balanced diet, with a high content of polyphenols, can bring important enhancement to the microbiota. Furthermore, considering the beneficial effects of polyphenols on the bacteria involved in metabolic syndrome, this approach could provide new foundations for their introduction in patients with acne, as the bacteria involved are the same. Therefore, a new holistic approach should include both the use of polyphenols and a balanced diet for improved health.
Author Contributions
Conceptualization, data curation, writing—original draft, writing—review and editing: S.I., S.N. and L.M., data curation, writing—original draft: R.C., S.P., L.C.P., G.R., S.U., R.M., F.S., V.M. (Valeria Mazza), M.S., J.M., E.S. and F.O., funding acquisition, supervision, writing—review and editing: C.M., supervision, writing—review and editing: S.M., C.T. and E.P., conceptualization, funding acquisition, supervision, writing—review and editing: V.M. (Vincenzo Mollace). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported by the Italian Ministry of Health (grant SG-2021-12375551); PRIN 2022 (grant code: 202273HF83); PRIN 2022 PNRR (grant code: P2022FAS5R); and grant PRODIGI “Finanziato dall’Unione europea-Next Generation EU, Missione 4 Componente 1 CUP B83C22002820006”. This study was also supported by the Italian Ministry of Health [Ricerca Corrente].
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Mahmud M.R., Akter S., Tamanna S.K., Mazumder L., Esti I.Z., Banerjee S., Akter S., Hasan M.R., Acharjee M., Hossain M.S., et al. Impact of gut microbiome on skin health: Gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes. 2022;14:2096995. doi: 10.1080/19490976.2022.2096995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garofalo C., Cristiani C.M., Ilari S., Passacatini L.C., Malafoglia V., Viglietto G., Maiuolo J., Oppedisano F., Palma E., Tomino C., et al. Fibromyalgia and Irritable Bowel Syndrome Interaction: A Possible Role for Gut Microbiota and Gut-Brain Axis. Biomedicines. 2023;11:1701. doi: 10.3390/biomedicines11061701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maiuolo J., Gliozzi M., Musolino V., Carresi C., Scarano F., Nucera S., Scicchitano M., Oppedisano F., Bosco F., Ruga S., et al. The Contribution of Gut Microbiota-Brain Axis in the Development of Brain Disorders. Front. Neurosci. 2021;15:616883. doi: 10.3389/fnins.2021.616883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sędzikowska A., Szablewski L. Human Gut Microbiota in Health and Selected Cancers. Int. J. Mol. Sci. 2021;22:13440. doi: 10.3390/ijms222413440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thursby E., Juge N. Introduction to the human gut microbiota. Biochem. J. 2017;474:1823–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maiuolo J., Carresi C., Gliozzi M., Mollace R., Scarano F., Scicchitano M., Macri R., Nucera S., Bosco F., Oppedisano F., et al. The Contribution of Gut Microbiota and Endothelial Dysfunction in the Development of Arterial Hypertension in Animal Models and in Humans. Int. J. Mol. Sci. 2022;23:3698. doi: 10.3390/ijms23073698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valdes A.M., Walter J., Segal E., Spector T.D. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:k2179. doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun J., Chen S., Zang D., Sun H., Sun Y., Chen J. Butyrate as a promising therapeutic target in cancer: From pathogenesis to clinic (Review) Int. J. Oncol. 2024;64:44. doi: 10.3892/ijo.2024.5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Vadder F., Kovatcheva-Datchary P., Goncalves D., Vinera J., Zitoun C., Duchampt A., Bäckhed F., Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 10.Portincasa P., Bonfrate L., Vacca M., De Angelis M., Farella I., Lanza E., Khalil M., Wang D.Q., Sperandio M., Di Ciaula A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022;23:1105. doi: 10.3390/ijms23031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patterson E., Ryan P.M., Cryan J.F., Dinan T.G., Ross R.P., Fitzgerald G.F., Stanton C. Gut microbiota, obesity and diabetes. Postgrad. Med. J. 2016;92:286–300. doi: 10.1136/postgradmedj-2015-133285. [DOI] [PubMed] [Google Scholar]
- 12.Stokes J.H., Pillsbury D.M. The effect on the skin of emotional and nervous states: iii. theoretical and practical consideration of a gastro-intestinal mechanism. Arch. Dermatol. Syphilol. 1930;22:962–993. doi: 10.1001/archderm.1930.01440180008002. [DOI] [Google Scholar]
- 13.Reddymasu S.C., Sostarich S., McCallum R.W. Small intestinal bacterial overgrowth in irritable bowel syndrome: Are there any predictors? BMC Gastroenterol. 2010;10:23. doi: 10.1186/1471-230X-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lombardo L., Foti M., Ruggia O., Chiecchio A. Increased incidence of small intestinal bacterial overgrowth during proton pump inhibitor therapy. Clin. Gastroenterol. Hepatol. 2010;8:504–508. doi: 10.1016/j.cgh.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Hacini-Rachinel F., Gheit H., Le Luduec J.B., Dif F., Nancey S., Kaiserlian D. Oral probiotic control skin inflammation by acting on both effector and regulatory T cells. PLoS ONE. 2009;4:e4903. doi: 10.1371/journal.pone.0004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polkowska-Pruszyńska B., Gerkowicz A., Krasowska D. The gut microbiome alterations in allergic and inflammatory skin diseases—An update. J. Eur. Acad. Dermatol. Venereol. 2020;34:455–464. doi: 10.1111/jdv.15951. [DOI] [PubMed] [Google Scholar]
- 17.Zouboulis C.C., Jourdan E., Picardo M. Acne is an inflammatory disease and alterations of sebum composition initiate acne lesions. J. Eur. Acad. Dermatol. Venereol. 2014;28:527–532. doi: 10.1111/jdv.12298. [DOI] [PubMed] [Google Scholar]
- 18.Andreadi A., Muscoli S., Tajmir R., Meloni M., Minasi A., Muscoli C., Ilari S., Mollace V., Della Morte D., Bellia A., et al. Insulin Resistance and Acne: The Role of Metformin as Alternative Therapy in Men. Pharmaceuticals. 2022;16:27. doi: 10.3390/ph16010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salem I., Ramser A., Isham N., Ghannoum M.A. The Gut Microbiome as a Major Regulator of the Gut-Skin Axis. Front. Microbiol. 2018;9:1459. doi: 10.3389/fmicb.2018.01459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vallianou N., Stratigou T., Christodoulatos G.S., Dalamaga M. Understanding the Role of the Gut Microbiome and Microbial Metabolites in Obesity and Obesity-Associated Metabolic Disorders: Current Evidence and Perspectives. Curr. Obes. Rep. 2019;8:317–332. doi: 10.1007/s13679-019-00352-2. [DOI] [PubMed] [Google Scholar]
- 21.Liu R., Hong J., Xu X., Feng Q., Zhang D., Gu Y., Shi J., Zhao S., Liu W., Wang X., et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 2017;23:859–868. doi: 10.1038/nm.4358. [DOI] [PubMed] [Google Scholar]
- 22.Zheng X., Chen T., Jiang R., Zhao A., Wu Q., Kuang J., Sun D., Ren Z., Li M., Zhao M., et al. Hyocholic acid species improve glucose homeostasis through a distinct TGR5 and FXR signaling mechanism. Cell Metab. 2021;33:791–803.e797. doi: 10.1016/j.cmet.2020.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Nucera S., Scarano F., Macrì R., Mollace R., Gliozzi M., Carresi C., Ruga S., Serra M., Tavernese A., Caminiti R., et al. The Effect of an Innovative Combination of Bergamot Polyphenolic Fraction and Cynara cardunculus L. Extract on Weight Gain Reduction and Fat Browning in Obese Mice. Int. J. Mol. Sci. 2023;25:191. doi: 10.3390/ijms25010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canfora E.E., Meex R.C.R., Venema K., Blaak E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019;15:261–273. doi: 10.1038/s41574-019-0156-z. [DOI] [PubMed] [Google Scholar]
- 25.Zhou H., Sun L., Zhang S., Zhao X., Gang X., Wang G. Evaluating the Causal Role of Gut Microbiota in Type 1 Diabetes and Its Possible Pathogenic Mechanisms. Front. Endocrinol. 2020;11:125. doi: 10.3389/fendo.2020.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Z., Huang S., Li T., Li N., Han D., Zhang B., Xu Z.Z., Zhang S., Pang J., Wang S., et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome. 2021;9:184. doi: 10.1186/s40168-021-01115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu L., Zhang J., Cheng Y., Zhu M., Xiao Z., Ruan G., Wei Y. Gut microbiota: A new target for T2DM prevention and treatment. Front. Endocrinol. 2022;13:958218. doi: 10.3389/fendo.2022.958218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan H.M., Zhao H.J., Guo D.Y., Zhu P.Q., Zhang C.L., Jiang W. Gut microbiota alterations in moderate to severe acne vulgaris patients. J. Dermatol. 2018;45:1166–1171. doi: 10.1111/1346-8138.14586. [DOI] [PubMed] [Google Scholar]
- 29.Belli M., Barone L., Longo S., Prandi F.R., Lecis D., Mollace R., Margonato D., Muscoli S., Sergi D., Federici M., et al. Gut Microbiota Composition and Cardiovascular Disease: A Potential New Therapeutic Target? Int. J. Mol. Sci. 2023;24:11971. doi: 10.3390/ijms241511971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jie Z., Xia H., Zhong S.L., Feng Q., Li S., Liang S., Zhong H., Liu Z., Gao Y., Zhao H., et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 2017;8:845. doi: 10.1038/s41467-017-00900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nesci A., Carnuccio C., Ruggieri V., D’Alessandro A., Di Giorgio A., Santoro L., Gasbarrini A., Santoliquido A., Ponziani F.R. Gut Microbiota and Cardiovascular Disease: Evidence on the Metabolic and Inflammatory Background of a Complex Relationship. Int. J. Mol. Sci. 2023;24:9087. doi: 10.3390/ijms24109087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bungau A.F., Radu A.F., Bungau S.G., Vesa C.M., Tit D.M., Endres L.M.J.B., Pharmacotherapy Oxidative stress and metabolic syndrome in acne vulgaris: Pathogenetic connections and potential role of dietary supplements and phytochemicals. Biomed. Pharmacother. 2023;164:115003. doi: 10.1016/j.biopha.2023.115003. [DOI] [PubMed] [Google Scholar]
- 33.Cheng H., Zhang D., Wu J., Liu J., Zhou Y., Tan Y., Feng W., Peng C. Interactions between gut microbiota and polyphenols: A mechanistic and metabolomic review. Phytomedicine. 2023;119:154979. doi: 10.1016/j.phymed.2023.154979. [DOI] [PubMed] [Google Scholar]
- 34.Caminiti R., Carresi C., Mollace R., Macrì R., Scarano F., Oppedisano F., Maiuolo J., Serra M., Ruga S., Nucera S., et al. The potential effect of natural antioxidants on endothelial dysfunction associated with arterial hypertension. Front. Cardiovasc. Med. 2024;11:1345218. doi: 10.3389/fcvm.2024.1345218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oliviero F., Scanu A., Zamudio-Cuevas Y., Punzi L., Spinella P. Anti-inflammatory effects of polyphenols in arthritis. J. Sci. Food Agric. 2018;98:1653–1659. doi: 10.1002/jsfa.8664. [DOI] [PubMed] [Google Scholar]
- 36.Bouarab-Chibane L., Forquet V., Lantéri P., Clément Y., Léonard-Akkari L., Oulahal N., Degraeve P., Bordes C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure-Activity Relationship) Models. Front. Microbiol. 2019;10:829. doi: 10.3389/fmicb.2019.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maiuolo J., Gliozzi M., Musolino V., Carresi C., Scarano F., Nucera S., Scicchitano M., Bosco F., Ruga S., Zito M.C., et al. From Metabolic Syndrome to Neurological Diseases: Role of Autophagy. Front. Cell Dev. Biol. 2021;9:651021. doi: 10.3389/fcell.2021.651021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maleki D.P., Sadoughi F., Asemi Z., Yousefi B. The role of polyphenols in overcoming cancer drug resistance: A comprehensive review. Cell Mol. Biol. Lett. 2022;27:1. doi: 10.1186/s11658-021-00301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nucera S., Ruga S., Cardamone A., Coppoletta A.R., Guarnieri L., Zito M.C., Bosco F., Macri R., Scarano F., Scicchitano M., et al. MAFLD progression contributes to altered thalamus metabolism and brain structure. Sci. Rep. 2022;12:1207. doi: 10.1038/s41598-022-05228-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahimlou M., Baghdadi G., Khodi A., Rahimi Z., Saki N., Banaei Jahromi N., Cheraghian B., Tavasolian R., Hosseini S.A. Polyphenol consumption and Nonalcoholic fatty liver disease risk in adults. Sci. Rep. 2024;14:6752. doi: 10.1038/s41598-024-57416-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krawczyk M., Burzynska-Pedziwiatr I., Wozniak L.A., Bukowiecka-Matusiak M. Impact of Polyphenols on Inflammatory and Oxidative Stress Factors in Diabetes Mellitus: Nutritional Antioxidants and Their Application in Improving Antidiabetic Therapy. Biomolecules. 2023;13:1402. doi: 10.3390/biom13091402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feldman F., Koudoufio M., Desjardins Y., Spahis S., Delvin E., Levy E. Efficacy of Polyphenols in the Management of Dyslipidemia: A Focus on Clinical Studies. Nutrients. 2021;13:672. doi: 10.3390/nu13020672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maiuolo J., Bulotta R.M., Ruga S., Nucera S., Macrì R., Scarano F., Oppedisano F., Carresi C., Gliozzi M., Musolino V., et al. The Postbiotic Properties of Butyrate in the Modulation of the Gut Microbiota: The Potential of Its Combination with Polyphenols and Dietary Fibers. Int. J. Mol. Sci. 2024;25:6971. doi: 10.3390/ijms25136971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ilari S., Proietti S., Russo P., Malafoglia V., Gliozzi M., Maiuolo J., Oppedisano F., Palma E., Tomino C., Fini M., et al. A Systematic Review and Meta-Analysis on the Role of Nutraceuticals in the Management of Neuropathic Pain in In Vivo Studies. Antioxidants. 2022;11:2361. doi: 10.3390/antiox11122361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El Gharras H. Polyphenols: Food sources, properties and applications–A review. Int. J. Food Sci. Technol. 2009;44:2512–2518. doi: 10.1111/j.1365-2621.2009.02077.x. [DOI] [Google Scholar]
- 46.Liu F., Shan S., Li H., Shi J., Hao R., Yang R., Li Z. Millet shell polyphenols prevent atherosclerosis by protecting the gut barrier and remodeling the gut microbiota in ApoE(-/-) mice. Food Funct. 2021;12:7298–7309. doi: 10.1039/D1FO00991E. [DOI] [PubMed] [Google Scholar]
- 47.Wan M.L.Y., Co V.A., El-Nezami H. Dietary polyphenol impact on gut health and microbiota. Crit. Rev. Food Sci. Nutr. 2021;61:690–711. doi: 10.1080/10408398.2020.1744512. [DOI] [PubMed] [Google Scholar]
- 48.Lu Y., Yuan X., Wang M., He Z., Li H., Wang J., Li Q. Gut microbiota influence immunotherapy responses: Mechanisms and therapeutic strategies. J. Hematol. Oncol. 2022;15:47. doi: 10.1186/s13045-022-01273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Open Med. 2009;3:e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson K.G., Rainer B.M., Antonescu C., Florea L., Mongodin E.F., Kang S., Chien A.L. Minocycline and Its Impact on Microbial Dysbiosis in the Skin and Gastrointestinal Tract of Acne Patients. Ann. Dermatol. 2020;32:21–30. doi: 10.5021/ad.2020.32.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deng Y., Wang H., Zhou J., Mou Y., Wang G., Xiong X. Patients with Acne Vulgaris Have a Distinct Gut Microbiota in Comparison with Healthy Controls. Acta Derm. Venereol. 2018;98:783–790. doi: 10.2340/00015555-2968. [DOI] [PubMed] [Google Scholar]
- 52.Manzhalii E., Hornuss D., Stremmel W. Intestinal-borne dermatoses significantly improved by oral application of Escherichia coli Nissle 1917. World J. Gastroenterol. 2016;22:5415–5421. doi: 10.3748/wjg.v22.i23.5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moreno-Indias I., Sánchez-Alcoholado L., Pérez-Martínez P., Andrés-Lacueva C., Cardona F., Tinahones F., Queipo-Ortuño M.I. Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients. Food Funct. 2016;7:1775–1787. doi: 10.1039/C5FO00886G. [DOI] [PubMed] [Google Scholar]
- 54.Vaiserman A.M., Koliada A.K., Marotta F. Gut microbiota: A player in aging and a target for anti-aging intervention. Ageing Res. Rev. 2017;35:36–45. doi: 10.1016/j.arr.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 55.Ni J., Wu G.D., Albenberg L., Tomov V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017;14:573–584. doi: 10.1038/nrgastro.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ben Meir Y.A., Nikbachat M., Portnik Y., Jacoby S., Adin G., Moallem U., Halachmi I., Miron J., Mabjeesh S.J. Effect of forage-to-concentrate ratio on production efficiency of low-efficient high-yielding lactating cows. Animal. 2021;15:100012. doi: 10.1016/j.animal.2020.100012. [DOI] [PubMed] [Google Scholar]
- 57.Zhang X., Zhao A., Sandhu A.K., Edirisinghe I., Burton-Freeman B.M. Red Raspberry and Fructo-Oligosaccharide Supplementation, Metabolic Biomarkers, and the Gut Microbiota in Adults with Prediabetes: A Randomized Crossover Clinical Trial. J. Nutr. 2022;152:1438–1449. doi: 10.1093/jn/nxac037. [DOI] [PubMed] [Google Scholar]
- 58.La Reau A.J., Suen G. TheRuminococci: Key symbionts of the gut ecosystem. J. Microbiol. 2018;56:199–208. doi: 10.1007/s12275-018-8024-4. [DOI] [PubMed] [Google Scholar]
- 59.Franzosa E.A., Sirota-Madi A., Avila-Pacheco J., Fornelos N., Haiser H.J., Reinker S., Vatanen T., Hall A.B., Mallick H., McIver L.J., et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 2019;4:293–305. doi: 10.1038/s41564-018-0306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meslier V., Laiola M., Roager H.M., De Filippis F., Roume H., Quinquis B., Giacco R., Mennella I., Ferracane R., Pons N., et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut. 2020;69:1258–1268. doi: 10.1136/gutjnl-2019-320438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li G., Xie C., Lu S., Nichols R.G., Tian Y., Li L., Patel D., Ma Y., Brocker C.N., Yan T., et al. Intermittent Fasting Promotes White Adipose Browning and Decreases Obesity by Shaping the Gut Microbiota. Cell Metab. 2017;26:672–685.e674. doi: 10.1016/j.cmet.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Magne F., Gotteland M., Gauthier L., Zazueta A., Pesoa S., Navarrete P., Balamurugan R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients. 2020;12:1474. doi: 10.3390/nu12051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Varela-Trinidad G.U., Domínguez-Díaz C., Solórzano-Castanedo K., Íñiguez-Gutiérrez L., Hernández-Flores T.J., Fafutis-Morris M. Probiotics: Protecting Our Health from the Gut. Microorganisms. 2022;10:1428. doi: 10.3390/microorganisms10071428. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.