Table 1.
Synthesis of 4a,5,8,8a-tetrahydro-1H-spiro[5,8-methanoquinazoline-2,3′-indoline]-2′,4(3H)-dione (3a).
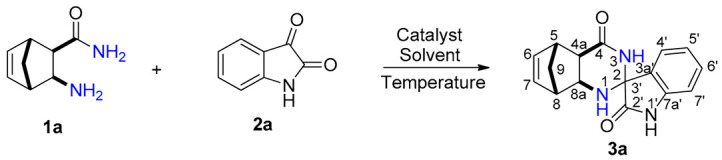
| |||||
|---|---|---|---|---|---|
| Entry | Catalyst | Solvent | Temp. (°C) | Time (h) | Yield a (%) |
| 1 | NH4Cl | EtOH | Rt b | 24 | 29 c |
| 2 | NH4Cl | EtOH | 78 | 12 | 37 c |
| 3 | NH4Cl | 2M2B d | 100 | 9 | 35 |
| 4 | LiOH | EtOH | Rt b | 72 | 10 |
| 5 | LiOH | EtOH | 78 | 72 | 21 |
| 6 | p-TsOH | EtOH | Rt b | 168 | – |
| 7 | p-TsOH | EtOH | 78 | 168 | – |
| 8 | Amberlyst 15 | EtOH | Rt b | 120 | 20 c |
| 9 | Amberlyst 15 | EtOH | 78 | 10 | 25 c |
| 10 | I2 | EtOH | Rt b | 215 | – |
| 11 | I2 | EtOH | 78 | 14 | 35 |
| 12 | Alum | Glycerol | Rt b | 168 | – |
| 13 | Alum | Glycerol | 100 | 5 | – |
| 14 | Alum | 2M2B d | Rt b | 72 | – |
| 15 | Alum | 2M2B d | 100 | 168 | – |
| 16 | Alum | EtOH | 78 | 5 | 42 |
| 17 | Alum | Water | rt | 168 | – e |
| 18 | Alum | Water | 100 | 8 | – e |
a Isolated yield after purification by flash chromatography. b Room temperature. c The reaction was not selective, since the product contained the mixture of two diastereomers according to the 1H-NMR spectrum. d 2M2B: 2-methyl-2-butanol. e The starting materials did not dissolve in water and there was no transformation.
