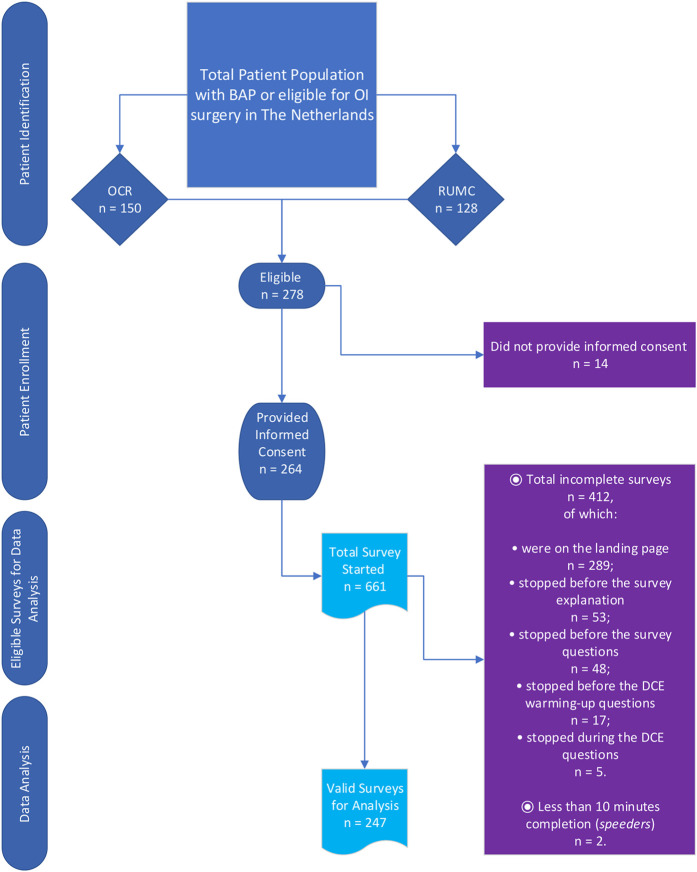
Fig. 3.
Study flowchart. Note that 264 patients provided informed consent; however, patients may have opened the survey, which was sent by e-mail with a personal link, multiple times resulting in a high number of surveys started (n = 661). However, only completed surveys were used for data analysis (n = 247). Reasons for incomplete surveys are provided in the purple exclusion box. BAP = bone-anchored prosthesis, OI = osseointegrated implant, OCR = Osseointegration Center Rotterdam, RUMC = Radboud University Medical Center, and DCE = discrete choice experiment.