FIG 4.
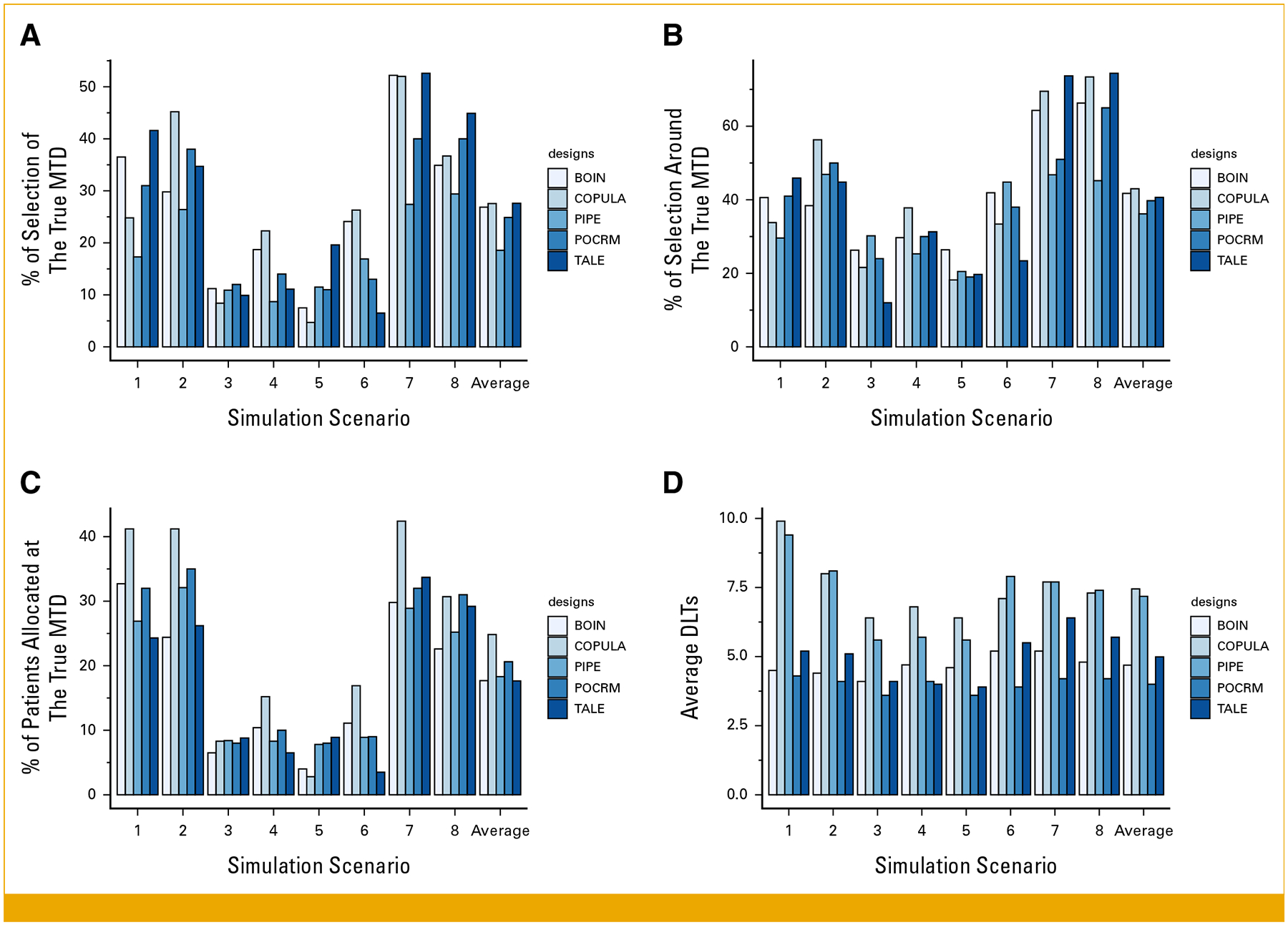
Comparison of the proposed TALE design and existing model-based or model-assisted methods. We focus on metrics a-d evaluated in scenarios 1–8. BOIN, Bayesian optimal interval design; DLTs, dose-limiting toxicities; MTD, maximum tolerated dose; PIPE, product of independent beta probabilities escalation; POCRM, continual reassessment method for partial ordering; TALE, Toxicity Adaptive Lists Design.
