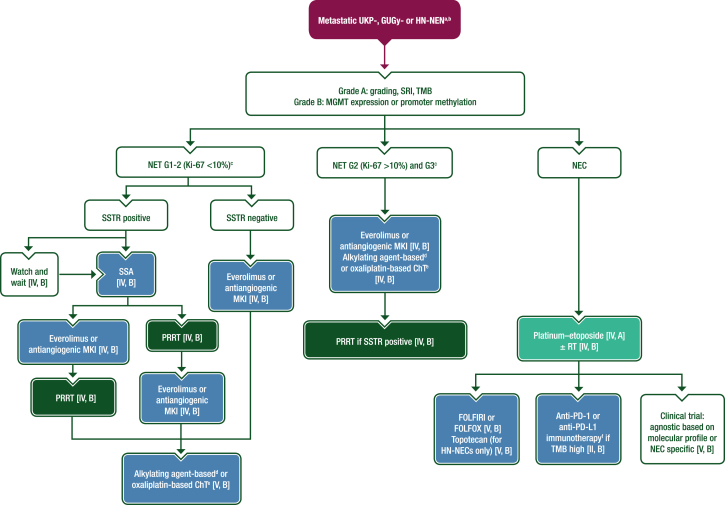
Figure 4.
Management of metastatic UKP-, GUGy- and HN-NENs. Purple: algorithm title; dark green: RT; blue: systemic anticancer therapy or their combination; turquoise: non-systemic anticancer therapies or combination of treatment modalities; white: other aspects of management and non-treatment aspects. 5-FU, 5-fluorouracil; ChT, chemotherapy; EMA, European Medicines Agency; FDA, Food and Drug Administration; FOLFIRI, leucovorin–5-fluorouracil–irinotecan; FOLFOX, leucovorin–5-fluorouracil–oxaliplatin; G, grade; GUGy, genitourinary or gynaecological; HN, head and neck; MGMT, O-6-methylguanine-DNA methyltransferase; MKI, multikinase inhibitor; NEC, neuroendocrine carcinoma; NEN, neuroendocrine neoplasm; NET, neuroendocrine tumour; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; PRRT, peptide receptor radionuclide therapy; R0, resection with no tumour at the margin; RT, radiotherapy; SRI, somatostatin receptor imaging; SSA, somatostatin analogue; SSTR, somatostatin receptor; TMB, tumour mutational burden; UKP, unknown primary. aAlways consider enrolment in a clinical trial [V, A]. bRecommendations for the management of metastatic UKP-, GUGy- and HN-NENs are based on the management of NENs of GEP or lung origin and therefore systemic treatments approved for GEP-NETs are used off-label in these settings. cSurgery is recommended if disease is amenable to R0 resection [V, A]. dDacarbazine, temozolomide or streptozocin plus 5-FU or capecitabine. eOxaliplatin plus 5-FU or capecitabine. fPembrolizumab is FDA approved (but not EMA approved) for the treatment of patients with unresectable or metastatic TMB high solid tumours that have progressed following prior treatment and have no alternative treatment options. No other anti-PD-1 or anti-PD-L1 agents are approved for use in patients with TMB high solid tumours.