Figure 1.
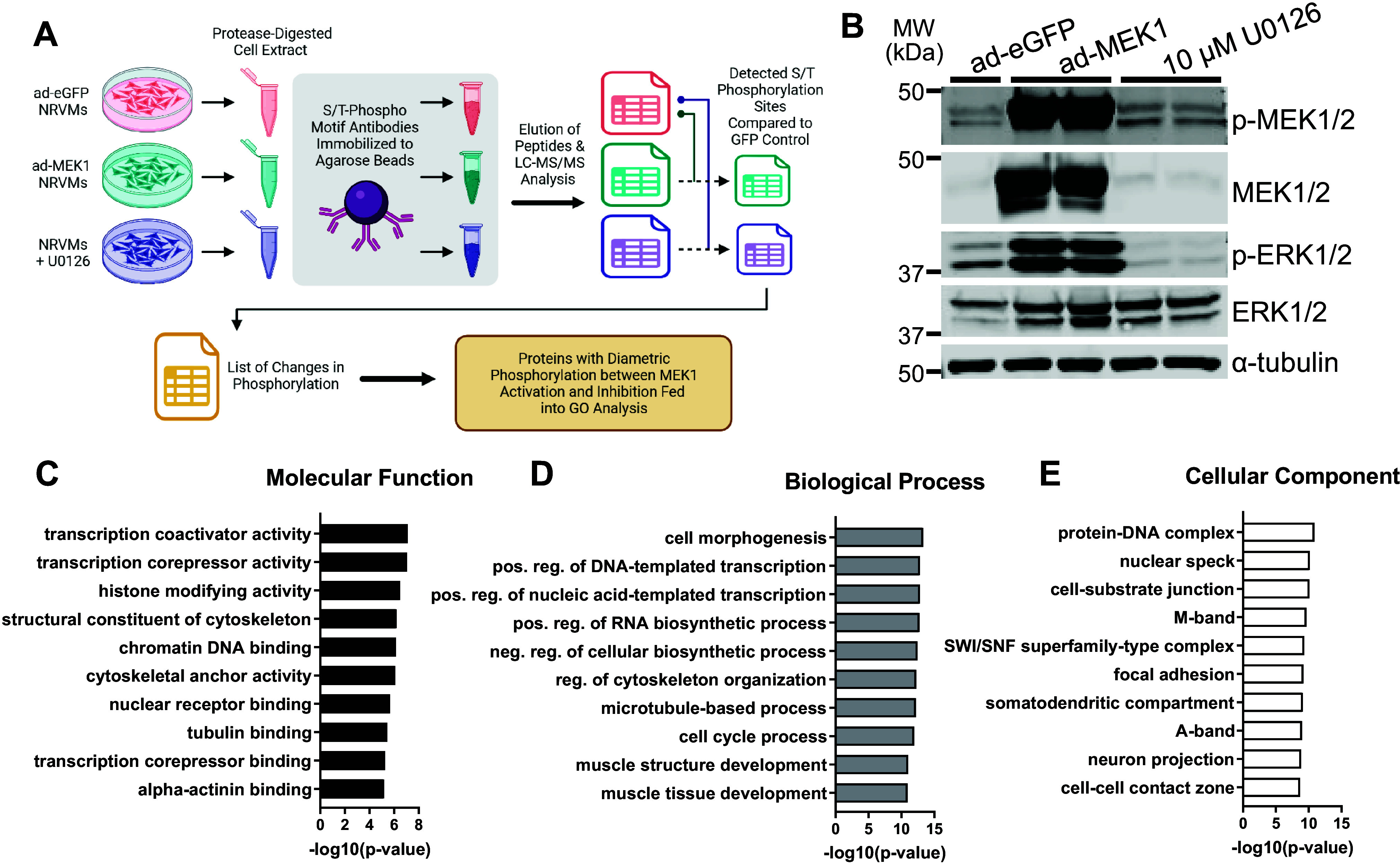
Phosphoproteomic data from analysis conducted on neonatal rat ventricular myocytes (NRVMs) with enhanced or inhibited MEK1-ERK1/2 signaling. A: schematic of the workflow from culture and treatment of NRVMs to the phosphoproteomic analysis (image was created with a licensed version of BioRrender.com). B: Western blots showing phosphorylated and total MEK1/2 and ERK1/2 with α-tubulin as a loading control for protein extracts of NRVMs subjected to enhanced green fluorescent protein (eGFP) adenovirus, activated MEK1 adenovirus, or U0126 MEK1 inhibitor, which served as inputs for the phosphoproteomics (n = 1–2/group with each sample representing a mixture of cells from both male and female hearts. Blots were repeated twice). C–E: graphs of the logarithmically transformed P values for gene ontology categories of the proteins identified in the screen which had phosphosites biphasically regulated under MEK1-ERK1/2 activation or inhibition for molecular function (C), biological process (D), and cellular component (E).
