Abstract
Aims
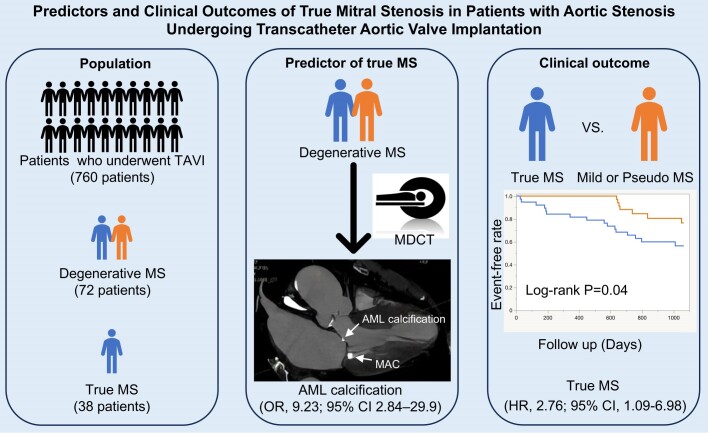
Predictors of true degenerative mitral stenosis (MS) in patients with aortic stenosis who underwent transcatheter aortic valve implantation (TAVI) remain unknown. This study aimed to investigate the predictors and prognostic value of true degenerative MS in this population.
Methods and results
We retrospectively reviewed the records of 760 consecutive patients who underwent TAVI. The mitral valve area (MVA) was assessed using transthoracic echocardiography, and mitral valve calcification was assessed using multi-detector computed tomography. MS was defined as an MVA of ≤2.0 cm², and true MS was defined as moderate or severe MS following TAVI. In our TAVI cohort, we identified 72 (9.5%) patients with degenerative MS. Among these, true MS was observed in 38 (52.7%) patients. Echocardiographic data showed that the true MS group had a significantly lower MVA and higher trans-mitral gradient. The severity of mitral annular calcification was not significantly different between the two groups; however, the true MS group had significantly more posterior mitral leaflet and anterior mitral leaflet (AML) calcification. Multivariable logistic regression analysis showed that AML calcification was the independent predictor of true MS [adjusted odds ratio, 9.23; 95% confidence interval (CI) 2.84–29.9]. True MS was independently associated with poor prognosis (adjusted hazard ratio, 2.76; 95% CI 1.09–6.98).
Conclusion
Approximately half of the patients with concomitant degenerative MS who underwent TAVI had true MS, which was associated with a poor prognosis. Computed tomographic analysis of AML calcification was useful for predicting true MS.
Keywords: aortic stenosis, mitral stenosis, transcatheter aortic valve implantation
Graphical Abstract
Graphical Abstract.
Introduction
The number of patients with degenerative mitral stenosis (MS) increases with age. Mitral annular calcification (MAC), the main cause of degenerative MS, is more prevalent in older adults, patients with chronic kidney disease, and individuals with multiple cardiovascular risk factors, such as hypertension, diabetes mellitus, dyslipidaemia, and smoking.1 These factors are often shared by patients with aortic stenosis (AS). Almost half of the patients with severe AS reportedly have MAC. Notably, almost half of the patients with severe AS also exhibit MAC, leading to a frequent comorbidity of degenerative MS and AS.2 Many of these patients are at high risk for open-heart surgery due to advanced age, frailty, and multiple comorbidities.3 Transcatheter aortic valve implantation (TAVI) has emerged as a treatment option for high-risk patients with severe AS. Approximately 10% of patients undergoing TAVI have concomitant MS.4 However, the co-occurrence of MS and AS complicates the assessment of the severity of each condition due to their interactions, and treatment strategies for these patients remain unestablished.5,6 A recent study reported that some AS patients with concomitant MS [trans-mitral gradient (TMG) ≥ 4 mmHg] have increased mitral valve area (MVA) after aortic valve replacement (AVR) (surgical AVR or TAVI), overestimating MS severity. Conversely, there are patients with ‘true MS’ whose MVA does not increase after AVR, and these patients are associated with poor prognosis.7
Previous studies have reported a poor prognosis in patients with concomitant severe MS undergoing TAVI.4 In patients with combined AS and MS, careful treatment selection is required, considering the severity of MS and patient’s general condition before undergoing intervention for AS.2,8 However, the predictors of true MS are not well known, and risk stratification in this population is important for determining treatment strategies. Therefore, this study aimed to investigate the predictors of true MS and their effect on the clinical outcomes of patients with degenerative MS undergoing TAVI.
Methods
Study population
This retrospective observational study included 760 consecutive patients aged 18 years and older with severe AS who underwent TAVI at St. Marianna University Hospital, Kawasaki, Japan, between January 2016 and December 2021. The inclusion criteria were patients diagnosed with degenerative MS, characterized by calcium deposition extending from the annulus to the apex without fusion of the mitral commissure. The exclusion criteria were as follows: (i) rheumatic MS, (ii) congenital heart disease, (iii) hypertrophic cardiomyopathy, (iv) moderate or severe aortic regurgitation, (v) moderate or severe mitral regurgitation, (vi) left ventricular (LV) outflow tract obstruction, and (vii) a heart rate of >100 bpm. The last six conditions were excluded because they affected the accuracy of MVA measurements using the continuity equation. This study was approved by the Institutional Ethics Committee of St. Marianna University School of Medicine (no. 6336), and the need for informed consent was waived owing to the retrospective nature of the study.
Transthoracic echocardiography and definitions of true MS
All patients underwent comprehensive two-dimensional and Doppler transthoracic echocardiography (TTE) according to the guidelines of the American Society of Echocardiography9,10,11 preoperatively, at discharge, at 1 year, and annually after TAVI. Because the TMG in this population must consider LV and left atrial compliance and may not reflect the exact severity of MS,12 this study defined an MVA of ≤2.0 cm² as MS. The MVA was measured using the continuity equation, and the severity of MS was defined as severe (≤1.0 cm²), moderate (1.0–1.5 cm²), or mild (1.5–2.0 cm²).13 In this study, a significant change was defined as a change of ≥0.1 cm² between pre-TAVI and post-TAVI discharge.14 True MS was defined as moderate or severe MS after TAVI.
Assessment of mitral valve calcification
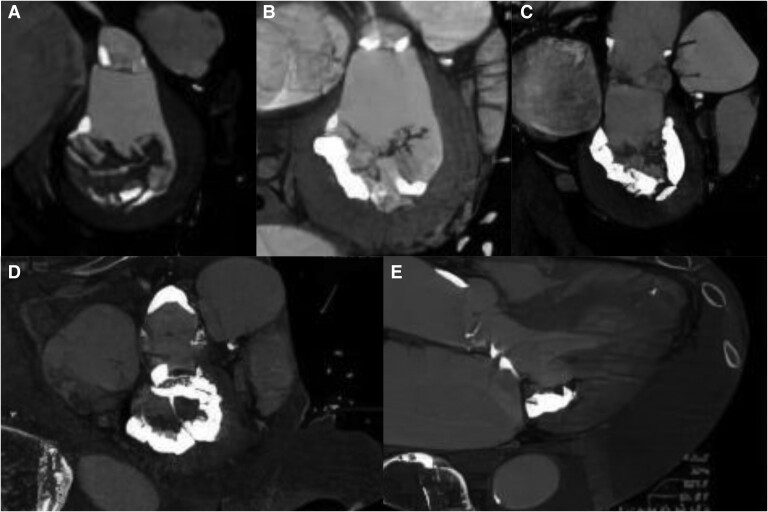
MAC was evaluated morphologically using multi-detector computed tomography (CT) (Aquilion One; Canon, Tokyo, Japan) before TAVI. CT data were processed for analysis using Ziostation2 software (Ziosoft, Tokyo, Japan). Three-dimensional analysis of this program was used to reconstruct the mitral valve in short-axis, long-axis, and commissural views, and the distribution of calcification was evaluated from multiple cross-sections. The severity of MAC was evaluated using the short-axis view and defined as the percentage of the area of calcification relative to the circumference of the mitral annulus: mild (<1/3), moderate (1/3–1/2), and severe (>1/2),15 as shown in Figure 1A–C. In addition, with reference to the cardiac CT-based assessment method for mitral valve calcification previously reported by Guerrero et al.,16 the commissures of the mitral valve were identified using the short-axis view (Figure 1D) and further assessed using the long-axis view (Figure 1E) to confirm anterior mitral leaflet (AML) and posterior mitral leaflet (PML) calcification.
Figure 1.
Qualitative evaluation of mitral valve calcification assessed using multi-detector CT. MAC severity was determined according to the visual circumferential involvement of the mitral annulus as follows: (A) mild (less than one-third of the annulus), (B) moderate (between one-third and one-half of the annulus), and (C) severe (more than half of the annulus). Valve leaflet calcification was identified by observing the mitral valve in two directions, the short axis (D) and long axis (E), to distinguish it from LV outflow tract calcification or MAC.
Data collection and clinical outcome
Baseline clinical data, procedural characteristics, and follow-up data were retrospectively collected from the hospital medical records. Routine clinical follow-ups were performed 1 month, 1 year, and annually after TAVI. The primary endpoint was a composite of all-cause death and heart failure hospitalization and stroke according to Valve Academic Research Consortium (VARC) 3 after TAVI.17 Clinical outcomes were collected using records from the institution’s integrated data system, documents provided by the referring physicians, and telephone interviews based on prior consent.
Statistical analyses
Continuous data were assessed using the Shapiro–Wilk test for the presence or absence of a normal distribution and are presented as the mean (standard deviation) when variables are normally distributed and as the median (interquartile range) when variables are not normally distributed. Categorical data are presented as frequencies or percentages. Continuous variables were compared between groups using the Student t-test or Wilcoxon rank-sum test, based on the normality of the data. The Student t-test was applied for normally distributed variables, while the Wilcoxon rank-sum test was used for non-normally distributed variables. Categorical data were compared between groups using the χ2 test. Logistic regression analysis was performed to identify variables associated with true MS. The independent variables included in the multivariate analysis were those considered to be associated with the outcomes based on the findings of previous studies and clinical judgement. The prognostic data were censored on 30 June 2024. Patients lost to follow-up were censored at the date of the last contact/follow-up; patients alive on 30 June 2024 were censored for event-free survival analysis. Event-free survival was defined as post-TAVI until the date of the primary endpoint. Event-free survival was estimated using the Kaplan–Meier method, and survival estimates were compared using the log-rank test. Cox regression analysis, both univariate and multivariate, was performed to assess the prognostic impact of true MS in this population. Specifically, the Society of Thoracic Surgeons score, a comprehensive clinical risk score, was included as a variable in both univariate and multivariate analyses. The proportional hazards assumption for the Cox model was tested and confirmed to be valid. The results are presented as odds ratios (ORs) [95% confidence intervals (CIs)] and hazard ratios (HRs) (95% CIs). Tests were two-sided, with P < 0.05 considered statistically significant. In cases where certain values were missing, a complete case analysis was performed. All statistical analyses were performed using JMP Pro 16 software (SAS Institute, Cary, NC, USA).
Results
Study population and baseline characteristics
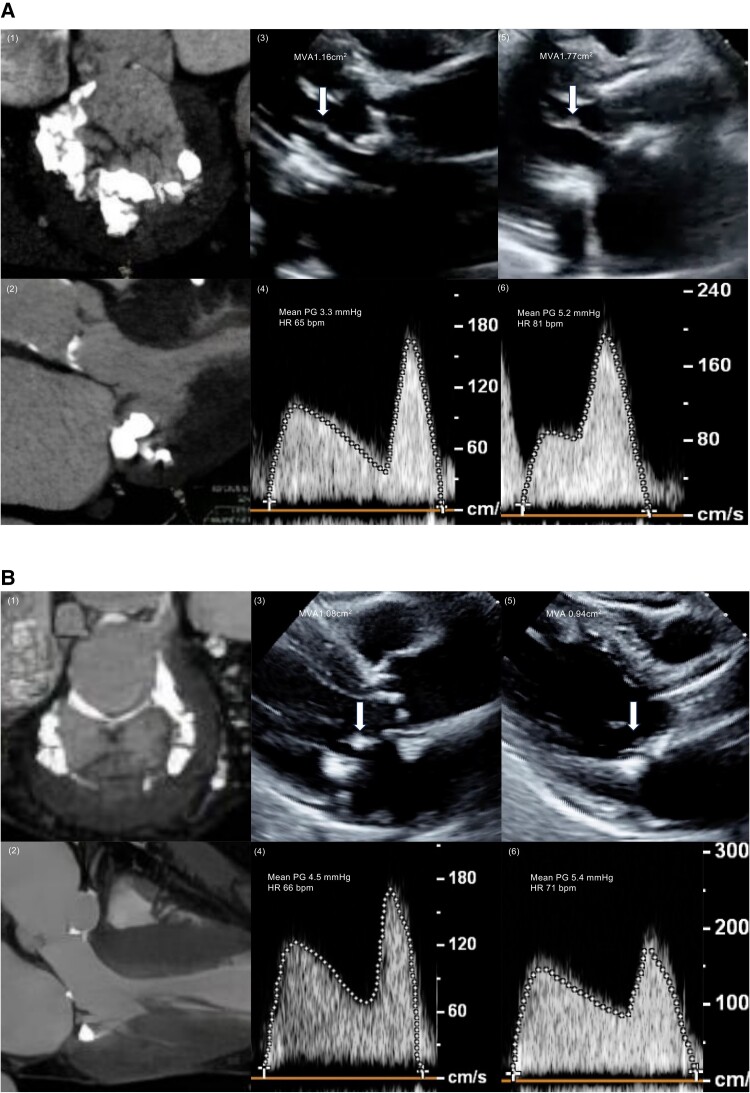
In the TAVI cohort, degenerative MS was observed in 72 (9.5%) patients. Of these 72 patients, 38 (52.7%) had true MS, while the remaining had pseudo- or mild MS. Baseline clinical characteristics are presented in Table 1. No significant differences were observed in the clinical characteristics between the two groups. TTE and CT characteristics are presented in Table 2. LV ejection fraction (LVEF), stroke volume (SV), and AS severity did not differ between the two groups. Compared with the mild or pseudo-MS group, the true MS group had a significantly lower MVA and higher TMG [MVA: 1.10 (0.90–1.31) vs. 1.41 (1.20–1.65) cm2, P < 0.001; TMG: 4.2 (3.6–5.9) vs. 3.3 (2.6–4.1) mmHg, P < 0.001]. Moreover, the true MS group had less mild MS and more severe MS, and no difference was observed in moderate MS [mild MS: 5 (13%) vs. 13 (38%), P = 0.03; moderate MS: 17 (45%) vs. 20 (59%), P = 0.16; severe MS: 16 (42%) vs. 1 (3%), P < 0.001]. Patients in the true MS group exhibited significantly higher values of diastolic function parameters, such as E-wave velocity, A-wave velocity, and E/e′ ratio [E-wave velocity: 128 (97–150) vs. 97 (75–116) cm/s, P < 0.001; A-wave velocity: 152 (133–170) vs. 128 (118–150) cm/s, P < 0.001; E/e′: 31.2 (24.0–40.2) vs. 25.6 (19.0–29.2), P = 0.004]. Although CT findings showed no difference in the severity of MAC between the two groups [moderate or severe MAC: 29 (76%) vs. 23 (68%), P = 0.41], the true MS group had more PML and AML calcification than the mild or pseudo-MS group [PML calcification: 30 (79%) vs. 19 (56%), P = 0.04; AML calcification: 26 (68%) vs. 7 (21%), P < 0.001]. Representative cases of true and pseudo-MS are shown in Figure 2.
Table 1.
Comparison of baseline characteristics
| True MS (n = 38) | Mild or pseudo-MS (n = 34) | P-value | |
|---|---|---|---|
| Age | 84 (82–86) | 84 (77–87) | 0.44 |
| Sex (male) | 7 (18%) | 9 (26%) | 0.41 |
| BMI, kg/m2 | 22.5 ± 3.5 | 21.8 ± 3.4 | 0.35 |
| BSA, m2 | 1.42 ± 0.14 | 1.44 ± 0.14 | 0.52 |
| NYHA Class III or IV | 12 (32%) | 17 (50%) | 0.11 |
| Clinical frailty scale | 4.0(3.0–5.0) | 4.0 (3.0–5.0) | 0.87 |
| High surgical risk (STS PROM ≥ 8) | 6 (16%) | 12 (35%) | 0.06 |
| Hypertension | 34 (89%) | 27 (79%) | 0.43 |
| Diabetes | 13 (39%) | 10 (26%) | 0.23 |
| Dyslipidaemia | 15 (41%) | 8 (24%) | 0.12 |
| Chronic kidney disease | 20 (57%) | 22 (65%) | 0.52 |
| Dialysis | 2 (5%) | 6 (18%) | 0.10 |
| Smoking | 7 (19%) | 11 (32%) | 0.21 |
| Chronic lung disease | 1 (3%) | 4 (12%) | 0.13 |
| Atrial fibrillation | 7 (18%) | 6 (18%) | 0.93 |
| Cerebrovascular disorder | 5 (15%) | 7 (21%) | 0.49 |
| ACE inhibitor/ARB | 18 (47%) | 11 (32%) | 0.19 |
| β-Blocker | 17 (45%) | 12 (35%) | 0.41 |
| Ca blocker | 19 (50%) | 18 (53%) | 0.80 |
| Statin | 14 (37%) | 14 (41%) | 0.71 |
| Hob, g/dL | 11.0 ± 1.3 | 11.4 ± 1.7 | 0.21 |
| Cr, mg/dL | 0.94 (0.71–1.14) | 0.86 (0.73–1.56) | 0.84 |
| eGFR, mL/min/1.73 m2 | 45.7 (35.4–60.1) | 47.4 (26.3–66.0) | 0.89 |
| LDL, mg/dL | 101 (89–119) | 100 (85–122) | 0.70 |
| HDL, mg/dL | 53 (44–65) | 59 (48–68) | 0.18 |
| HbA1c, % | 5.7 (5.6–6.1) | 5.7 (5.5–6.4) | 0.68 |
| NT-proBNP, pg/mL | 1500 (841–3407) | 2219 (745–11 433) | 0.40 |
| Valve type (balloon expandable) | 29 (76%) | 27 (79%) | 0.75 |
| Valve size | 0.24 | ||
| 20 mm | 6 (16%) | 1 (3%) | 0.053 |
| 23 mm | 20 (53%) | 18 (53%) | 0.98 |
| 26 mm | 11 (29%) | 14 (41%) | 0.28 |
| 29 mm | 1 (3%) | 1 (3%) | 0.94 |
| Use of circulatory assist device | 1 (3%) | 3 (9%) | 0.24 |
| Intraoperative complications | 1 (3%) | 1 (3%) | 0.94 |
Data are presented as the n (%), median (interquartile range), or mean (standard deviation, SD).
ACE, angiotensin-converting enzyme; BMI, body mass index; BSA, body surface area; Ca, calcium; Cr, creatinine; eGFR, estimated glomerular filtration rate; Hob, haemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; STS PROM, Society of Thoracic Surgeon Predicted Risk of Mortality; TAVI, transcatheter aortic valve implantation.
Table 2.
Comparison of echocardiographic and CT data
| True MS (n = 38) | Mild or pseudo-MS (n = 34) | P-value | |
|---|---|---|---|
| SBP, mmHg | 129 (114–154) | 131 (121–153) | 0.56 |
| DBP, mmHg | 61 (54–76) | 64 (58–78) | 0.16 |
| HR, bpm | 70 (62–75) | 73 (64–79) | 0.45 |
| LVEF, % | 68 (62–73) | 64 (55–71) | 0.06 |
| SV, mL | 51 (44–61) | 52 (47–59) | 0.88 |
| SV index, mL/m2 | 37.0 (31.0–41.0) | 37.0 (31.0–42.3) | 0.95 |
| LVDd, mm | 41 (38–44) | 42 (39–51) | 0.15 |
| LVDs, mm | 26 (23–27) | 29 (24–34) | 0.03 |
| IVS, mm | 11 (9–12) | 10 (9–12) | 0.33 |
| PWD, mm | 11 (9–12) | 10 (9–12) | 0.73 |
| LAD, mm | 43 (39–46) | 41 (35–45) | 0.17 |
| AVA, cm2 | 0.49 (0.41–0.65) | 0.54 (0.42–0.64) | 0.52 |
| AVA index, cm2/m2 | 0.37 (0.29–0.45) | 0.39 (0.30–0.44) | 0.50 |
| Peak V, m/s | 4.4 (3.8–4.9) | 4.4 (3.6–4.9) | 0.85 |
| Peak PG, mmHg | 77 (56–94) | 79 (53–96) | 0.85 |
| Mean PG, mmHg | 43 (34–55) | 42 (30–56) | 0.70 |
| MVA, cm2 | 1.10 (0.90–1.31) | 1.41 (1.20–1.65) | <0.001 |
| MS severity | <0.001 | ||
| Mild | 5 (13%) | 13 (38%) | 0.03 |
| Moderate | 17 (45%) | 20 (59%) | 0.16 |
| Severe | 16 (42%) | 1 (3%) | <0.001 |
| TMG, mmHg | 4.2 (3.6–5.9) | 3.3 (2.6–4.1) | <0.001 |
| E-wave velocity, cm/s | 128 (97–150) | 97 (75–116) | 0.005 |
| A-wave velocity, cm/s | 152 (133–170) | 128 (118–150) | 0.001 |
| e′-wave velocity, cm/s | 3.5 (3.0–4.0) | 3.8 (3.0–4.5) | 0.61 |
| E/A | 0.73 (0.58–1.00) | 0.72 (0.60–0.84) | 0.45 |
| E/e′ | 31.2 (24.0–40.2) | 25.6 (19.0–29.2) | 0.004 |
| LAVI, mL/m2 | 63.6 (49.2–79.7) | 53.6 (44.0–72.4) | 0.07 |
| TRPG, mmHg | 31 (26–39) | 34 (24–41) | 0.71 |
| Moderate or severe TR | 8 (21%) | 5 (15%) | 0.48 |
| Agatston score of aortic valve | 1539 (776–2193) | 1761 (1042–2846) | 0.21 |
| Moderate or severe MAC, % | 29 (76%) | 23 (68%) | 0.41 |
| PML calcification, % | 30 (79%) | 19 (56%) | 0.04 |
| AML calcification, % | 26 (68%) | 7 (21%) | <0.001 |
Data are presented as the n (%) or median (interquartile range). Due to the presence of atrial fibrillation patients in whom A-wave could not be measured, A-wave velocity and E/A had missing values in 16 cases (22%).
AML, anterior mitral leaflet; AS, aortic stenosis; AV, aortic valve; AVA, aortic valve area; DBP, diastolic blood pressure; HR, heart rate; IVS, interventricular septum; LAD, left atrial; LAVI, left atrial volume index; LVDd, left ventricular diastolic diameter; LVDs, left ventricular systolic diameter; LVEF, left ventricular ejection fraction; MAC, mitral annular calcification; MVA, mitral valve area; PG, pressure gradient; PML, posterior mitral leaflet; PWD, posterior wall diameter; RVSP, right ventricular systolic pressure; SBP, systolic blood pressure; SV, stroke volume.
Figure 2.
Representative cases of the true and pseudo-MS. (A) A typical case of the pseudo-MS group: CT shows severe MAC (1) but no calcification on the AML (2). TTE shows a MVA of 1.16 cm² and a mean pressure gradient (PG) of 3.3 mmHg before TAVI (3, 4). In this case, the AML opening was significantly increased after TAVI (3, 5), leading to an increase in MVA to 1.77 cm² and a slight increase in the mean PG to 5.2 mmHg after TAVI (5, 6). (B) A typical case of the true MS group: in addition to severe MAC, AML calcification was also observed (2). TTE showed that AML opening did not change before and after TAVI (3, 5), leading to no MVA increase and a slight increase in the mean PG after TAVI (4, 6).
Changes in TTE findings after TAVI
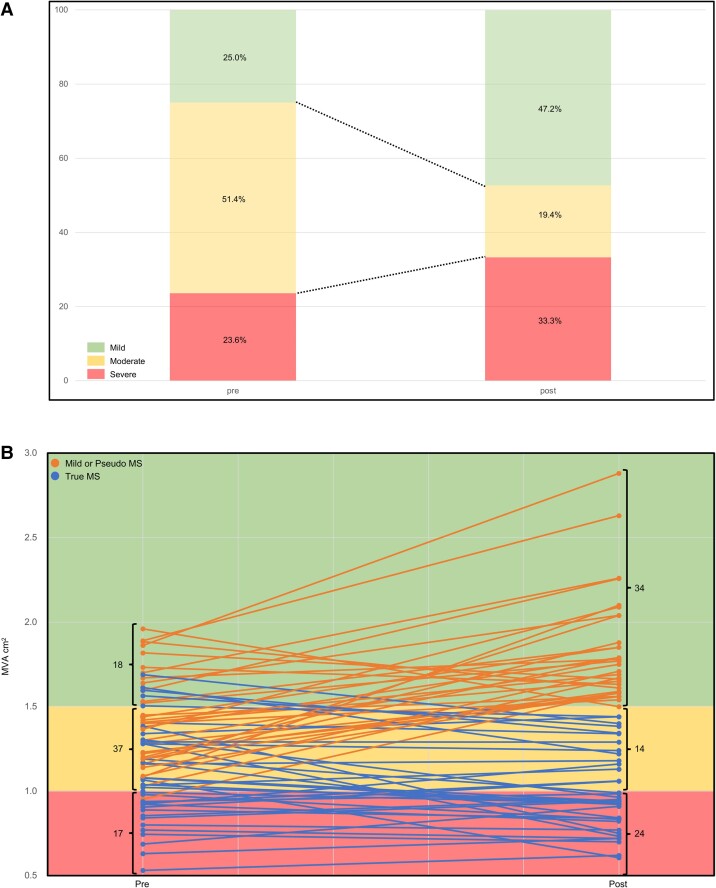
Table 3 presents the changes in the main TTE findings in both groups before and after TAVI. Compared with the mild or pseudo-MS group, the true MS group had smaller SV after TAVI and a smaller change from pre-TAVI to post-TAVI discharge [SV: 49.5 (43.0–58.0) vs. 61.0 (52.0–72.3) mL, P < 0.001; ΔSV: −3.5 (−7.4–1.0) vs. 11.0 (−2.5–19.0) mL, P < 0.001]. The true MS group also had a smaller effective orifice area index (EOAi) and ΔEOAi [EOAi: 0.81 (0.71–0.96) vs. 1.10 (0.88–1.37) cm², P < 0.001; ΔEOAi: 0.56 (0.47–0.68) vs. 0.83 (0.64–1.13) mL, P < 0.001]. The mild or pseudo-MS group had higher TMG, tricuspid regurgitation pressure gradient (TRPG), and ΔTRPG [TMG: 4.8 (3.3–6.4) vs. 3.8 (3.0–5.0) mmHg, P = 0.03; TRPG: 32.7 (28.2–43.5) vs. 28.9 (26.0–33.4), P = 0.03; ΔTRPG: 2.7 (−3.4–6.7) vs. −1.7 (−10.3–2.2) mmHg, P = 0.02]. In some patients undergoing TAVI, the severity of MS changed pre- and post-TAVI. Figure 3A shows the change in percentage of each severity, and Figure 3B shows the change in the number of cases of each severity and the change in MVA in each case.
Table 3.
Echocardiographic changes after TAVI
| True MS (n = 38) | Mild or pseudo-MS (n = 34) | P-value | |
|---|---|---|---|
| SV, mL | 49.5 (43.0–58.0) | 61.0 (52.0–72.3) | <0.001 |
| ΔSV, mL | −3.5 (−7.4–1.0) | 11.0 (−2.5–19.0) | <0.001 |
| EOA, cm2 | 1.14 (1.01–1.28) | 1.61 (1.31–1.91) | <0.001 |
| ΔEOA, cm2 | 0.81 (0.60–0.94) | 1.23 (0.90–1.54) | <0.001 |
| EOAi, cm2/m2 | 0.81 (0.71–0.96) | 1.10 (0.88–1.37) | <0.001 |
| ΔEOAi, cm2/m2 | 0.56 (0.47–0.68) | 0.83 (0.64–1.13) | <0.001 |
| Max velocity, m/s | 2.2 (1.9–2.5) | 2.0 (1.8–2.4) | 0.31 |
| Peak PG, mmHg | 19.6 (14.2–24.0) | 16.0 (12.9–22.4) | 0.31 |
| Mean PG, mmHg | 11.0 (8.0–13.2) | 8.2 (7.0–11.3) | 0.13 |
| MVA, cm2 | 0.94 (0.81–1.19) | 1.75 (1.56–2.04) | <0.001 |
| ΔMVA, cm2 | −0.06 (−0.21–0.09) | 0.40 (0.25–0.65) | <0.001 |
| ΔMVA ≥0.1 cm2 | 4 (11%) | 30 (88%) | <0.001 |
| TMG, mmHg | 4.8 (3.3–6.4) | 3.8 (3.0–5.0) | 0.03 |
| ΔTMG, mmHg | 0.5 (−0.3–1.4) | 0.4 (−0.4–1.1) | 0.78 |
| TRPG, mmHg | 32.7 (28.2–43.5) | 28.9 (26.0–33.4) | 0.03 |
| ΔTRPG, mmHg | 2.7 (−3.4–6.7) | −1.7 (−10.3–2.2) | 0.02 |
| Moderate or severe TR | 8 (22%) | 5 (15%) | 0.42 |
Data are presented as the n (%) or median (interquartile range). The symbol ‘Δ’ stands for ‘change’.
EOA, effective orifice area; EOAi, effective orifice area index; MVA, mitral valve area; PG, pressure gradient; SV, stroke volume; TMG, trans-mitral gradient; TR, tricuspid regurgitation; TRPG, tricuspid regurgitation pressure gradient.
Figure 3.
Changes in MS pre- and post-TAVI. (A) Change in percentage of each severity. (B) Change in the number of cases of each severity and the change in MVA in each case.
Factors associated with true MS
Univariate and multivariate logistic regression analyses were performed to identify independent factors statistically associated with true MS before TAVI (Table 4). The multivariate analysis identified a statistically significant association between true MS and a smaller MVA (adjusted OR, 1.29, 95% CI = 1.02–1.63). Additionally, AML calcification was identified as an independent factor associated with true MS (adjusted OR, 9.23; 95% CI = 2.84–29.9, P < 0.001).
Table 4.
Variables associated with true MS
| Univariate | Multivariate: Model 1 | Multivariate: Model 2 | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value | |
| Echocardiographic variables | ||||||
| SV index per 1 mL/m2 | 1.00 (0.95–1.05) | 0.88 | ||||
| MVA per 0.1 cm2a | 1.45 (1.19–1.76) | <0.001 | 1.29 (1.02–1.63) | 0.02 | ||
| TMG per 1 mmHg | 1.81 (1.20–2.72) | <0.001 | 1.40 (0.86–2.27) | 0.14 | ||
| DVI (MV VTI/LVOT VTI) ≧ 2.5 | 3.47 (0.94–12.8) | 0.06 | ||||
| E-wave velocity, cm/s | 1.02 (1.01–1.04) | 0.009 | 1.00 (0.98–1.03) | 0.54 | ||
| E/e′ | 1.09 (1.02–1.15) | 0.007 | 1.01 (0.93–1.10) | 0.78 | ||
| LAVI, mL/m2 | 1.02 (0.99–1.05) | 0.06 | ||||
| TRPG per 1 mmHg | 1.00 (0.96–1.05) | 0.85 | ||||
| CT variables | ||||||
| Moderate or severe MAC | 1.54 (0.55–4.35) | 0.41 | 0.48 (0.12–1.81) | 0.27 | ||
| PML calcification | 2.96 (1.05–8.32) | 0.04 | 2.91 (0.83–10.2) | 0.10 | ||
| AML calcification | 8.36 (2.85–24.5) | <0.001 | 9.23 (2.84–29.9) | <0.001 | ||
Model 1 was adjusted for the MVA, TMG, E-wave velocity, and E/e′. Model 2 was adjusted for moderate and severe MAC, PML, and AML calcification.
AML, anterior mitral leaflet; CI, confidence interval; CT, computed tomography; DVI, Doppler velocity index; LAVI, left atrial volume index; LVOT, left ventricular outflow tract; MAC, mitral annular calcification; MV, mitral valve; MVA, mitral valve area; OR, odds ratio; PML, posterior mitral leaflet; SV, stroke volume; TMG, trans-mitral gradient; TRPG, tricuspid regurgitation pressure gradient; VTI, velocity–time integral.
aA decrease per unit.
Clinical outcomes
During the 3-year follow-up after TAVI, 24 events were observed in the entire cohort: 12 (16.7%) patients died, 9 (12.5%) were hospitalized for heart failure, and 3 (4.2%) had strokes. The true MS group had a significantly lower event-free survival rate at 3 years compared with the mild or pseudo-MS group (56.4 ± 8% vs. 76.5 ± 8%, log-rank P = 0.04). In a multivariate Cox regression analysis, true MS was identified as an independent poor prognostic factor in this population (HR 2.76, 95% CI 1.09–6.98, P = 0.03; Figure 4). The detailed results of the Cox regression analysis are presented in Supplementary data online, Table S1. We performed an additional analysis including a reference group of patients undergoing TAVI without MS. The Kaplan–Meier curve is presented in Supplementary data online, Figure S1. In a multivariate Cox regression analysis, true MS was identified as an independent poor prognostic factor (HR 1.94, 95% CI 1.15–3.27, P = 0.01; Supplementary data online, Table S2). However, mild or pseudo-MS was not associated with poor prognosis.
Figure 4.
Event-free survival curve for the composite endpoint of all-cause death, heart failure hospitalization, and stroke. *Adjusted for Society of Thoracic Surgeon Predicted Risk of Mortality. CI, confidence interval; HR, hazard ratio; MS, mitral stenosis.
Discussion
Concomitant degenerative MS in patients undergoing TAVI presents a unique clinical challenge. The interaction between AS and MS complicates the accurate assessment of the severity of each condition, which, in turn, affects treatment strategies. The major findings of this study, which examined the recent focus on degenerative MS in the TAVI cohort, are as follows: (i) degenerative MS was present in 9% of the entire TAVI cohort, indicating that it is not uncommon; (ii) among patients with concomitant degenerative MS undergoing TAVI, approximately half had true MS; (iii) this study revealed the rate and extent of changes in MS severity following TAVI, attributable to the haemodynamic improvements induced by the procedure; (iv) true MS was associated with a poor prognosis; and (v) AML calcification on CT was useful for predicting true MS.
Predictors of true MS
Our analysis identified AML calcification as a significant predictor of true MS. This finding underscores the role of CT imaging in the comprehensive evaluation of mitral valve morphology before TAVI. The detailed assessment of mitral valve calcification using multi-detector CT allowed for the differentiation between true and pseudo-MS. Echocardiographic assessment of mitral valve characteristics in patients with degenerative MS is difficult because of calcification artefacts. Therefore, to identify true MS in patients with degenerative MS undergoing TAVI, we investigated not only echocardiographic findings but also CT findings before the TAVI procedure. Specifically, AML calcification was significantly more prevalent in the true MS group than in the mild or pseudo-MS group. There was a considerable difference in that patients with true MS exhibited significantly higher TMG and poorer clinical outcomes. Previous studies have reported a correlation between AML calcification and the MVA in patients with degenerative MS.18 Degenerative MS patients with AML calcification also reportedly had increased TMG and decreased MVA after TAVI.19 In our TAVI cohort, we observed improved AML mobility after TAVI in typical cases of mild or pseudo-MS; however, this phenomenon was not observed in typical patients with true MS (Figure 2). In addition, the ΔSV was associated with changes in the MVA before and after TAVI. These observations may be explained by the haemodynamic changes caused by TAVI and the fact that the AML has a longer valve length than the PML and is composed of a single scallop, unlike the PML, which comprises three scallops.
Clinical outcomes
True MS was associated with a significantly lower event-free survival rate at 3 years post-TAVI compared with mild or pseudo-MS. This finding aligns with that of previous studies that have reported poor prognosis in patients with severe MS undergoing TAVI.4,8,20 The higher TMG and smaller EOAi observed in the true MS group post-TAVI likely contributed to the adverse outcomes. The persistence of significant MS despite successful TAVI emphasizes the need for careful preoperative assessment and post-procedural monitoring.
Clinical implications
In cases of combined MS and AS, it is often difficult to determine which condition has a greater impact on the patient’s clinical presentation. When there is a discrepancy between the severity of symptoms and the apparent degree of stenosis, stress echocardiography is recommended for assessing MS.6 There is evidence that exercise-induced pulmonary hypertension, as evaluated by stress echocardiography, serves as a prognostic factor in MS patients,21 highlighting its potential value in such cases. However, the role of stress echocardiography in patients with both MS and AS remains unclear, and future studies and data accumulation are necessary. It is also important to recognize the limitations of stress echocardiography, particularly in patients with severe comorbidities or in cases where the presence of multiple valve diseases complicates interpretation. The identification of true MS has significant implications in clinical practice. In our study, some patients with degenerative MS who underwent TAVI showed a change in the severity of their MS after TAVI, masking the preoperative severity. Notably, this change was more pronounced in moderate MS, which is considered clinically significant MS.6 Given the poor prognosis associated with true MS, it is essential to incorporate detailed imaging analyses, such as CT, into the preoperative assessment of patients with AS undergoing TAVI. By identifying patients with true MS during pre-TAVI planning using echocardiography and CT, physicians can better stratify risks and tailor management strategies to improve outcomes. For example, the presence of AML calcification could prompt the consideration of more aggressive or alternative interventions to address both AS and MS.
Study limitations
This study had some limitations. First, this study used MVA derived from the continuity equation. Given the inherent measurement error in this method, low cut-off values may fall within the range of observational error. Therefore, we conducted a sensitivity analysis using a ΔMVA of 0.2 cm² as the definition of true MS without considering the severity of MS (see Supplementary data online, Figure S2). The results confirmed that true MS remained an independent poor prognostic factor at this cut-off (HR, 2.91; 95% CI = 1.07–7.87, P = 0.04). The primary result of this study, that AML calcification is an independent factor associated with true MS, was also upheld (adjusted OR 10.3, 95% CI =2.99–35.3, P < 0.001). These findings support the importance of our conclusions. Second, it was a retrospective observational study, which may have introduced a selection bias. Third, this study was conducted at a single centre and included a relatively small sample size, which may limit the generalizability of our findings. Fourth, the follow-up period, although relatively long, may not have captured all long-term outcomes. Future prospective multicentre studies are needed to validate our findings and explore the long-term impact of true MS in patients who have undergone TAVI.
Conclusions
Approximately half of the patients with concomitant degenerative MS undergoing TAVI had true MS, which was associated with a poor prognosis. CT analysis of AML calcification was useful for predicting true MS.
Supplementary Material
Contributor Information
Mitsuki Yamaga, Department of Cardiology, St. Marianna University School of Medicine, 2-16-1, Sugao, Miyamae-ku, Kawasaki 216-8511, Japan; Department of Cardiology, Mishuku Hospital, Tokyo, Japan.
Masaki Izumo, Department of Cardiology, St. Marianna University School of Medicine, 2-16-1, Sugao, Miyamae-ku, Kawasaki 216-8511, Japan.
Yukio Sato, Department of Cardiology, St. Marianna University School of Medicine, 2-16-1, Sugao, Miyamae-ku, Kawasaki 216-8511, Japan.
Tatsuro Shoji, Department of Cardiology, St. Marianna University School of Medicine, 2-16-1, Sugao, Miyamae-ku, Kawasaki 216-8511, Japan.
Daisuke Miyahara, Department of Cardiology, St. Marianna University School of Medicine, 2-16-1, Sugao, Miyamae-ku, Kawasaki 216-8511, Japan.
Yoshikuni Kobayashi, Department of Cardiology, St. Marianna University School of Medicine, 2-16-1, Sugao, Miyamae-ku, Kawasaki 216-8511, Japan.
Takahiko Kai, Department of Cardiology, St. Marianna University School of Medicine, 2-16-1, Sugao, Miyamae-ku, Kawasaki 216-8511, Japan.
Taishi Okuno, Department of Cardiology, St. Marianna University School of Medicine, 2-16-1, Sugao, Miyamae-ku, Kawasaki 216-8511, Japan.
Shingo Kuwata, Department of Cardiology, St. Marianna University School of Medicine, 2-16-1, Sugao, Miyamae-ku, Kawasaki 216-8511, Japan.
Masashi Koga, Department of Cardiology, St. Marianna University School of Medicine, 2-16-1, Sugao, Miyamae-ku, Kawasaki 216-8511, Japan.
Yasuhiro Tanabe, Department of Cardiology, St. Marianna University School of Medicine, 2-16-1, Sugao, Miyamae-ku, Kawasaki 216-8511, Japan.
Yoshihiro J Akashi, Department of Cardiology, St. Marianna University School of Medicine, 2-16-1, Sugao, Miyamae-ku, Kawasaki 216-8511, Japan.
Supplementary data
Supplementary data are available at European Heart Journal – Imaging Methods and Practice online.
Consent
The study protocol was approved by the Ethics Committee of St. Marianna University School of Medicine (Approval No. 6336). The requirement for informed consent was waived due to the retrospective nature of the study.
Funding
This study did not receive any specific funding.
Data availability
The data underlying this article were provided by St. Marianna University Hospital. Data will be shared on request to the corresponding author with permission of St. Marianna University Hospital.
Lead author biography

Mituski Yamaga is a cardiologist currently enrolled in the Graduate School of Medicine at St. Marianna University School of Medicine in Kawasaki, Japan. His research focuses on cardiovascular imaging, including echocardiography and CT, as well as structural heart disease.
References
- 1. Kato N, Padang R, Scott CG, Guerrero M, Pislaru SV, Pellikka PA. The natural history of severe calcific mitral stenosis. J Am Coll Cardiol 2020;75:3048–57. [DOI] [PubMed] [Google Scholar]
- 2. Abramowitz Y, Kazuno Y, Chakravarty T, Kawamori H, Maeno Y, Anderrson D et al. Concomitant mitral annular calcification and severe aortic stenosis: prevalence, characteristics and outcome following transcatheter aortic valve replacement. Euro Heart J 2017;38:1194–203. [DOI] [PubMed] [Google Scholar]
- 3. Sud K, Agarwal S, Parashar A, Raza MQ, Patel K, Min D et al. Degenerative mitral stenosis: unmet need for percutaneous interventions. Circulation 2016;133:1594–604. [DOI] [PubMed] [Google Scholar]
- 4. Joseph L, Bashir M, Xiang Q, Yerokun BA, Matsuoka RA, Vemulapalli S et al. Prevalence and outcomes of mitral stenosis in patients undergoing transcatheter aortic valve replacement: findings from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapies Registry. JACC Cardiovasc Interv 2018;11:693–702. [DOI] [PubMed] [Google Scholar]
- 5. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP III, Gentile F et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021;143:e35–71. [DOI] [PubMed] [Google Scholar]
- 6. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease: developed by the task force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Euro Heart J 2022;43:561–632. [Google Scholar]
- 7. Kato N, Padnag R, Pislaru C, Miranda WR, Hoshina M, Shibayama K et al. Hemodynamics and prognositic impact of concomitant mitral stenosis in patients undergoing surgical or tanscatheter aortic valve replacement for aortic stenosis. Circulation 2019;140:1251–60. [DOI] [PubMed] [Google Scholar]
- 8. Asami M, Windecker S, Praz F, Lanz J, Hunziker L, Rothenbühler M et al. Transcatheter aortic valve replacement in patients with concomitant mitral stenosis. Eur Heart J 2019;40:1342–51. [DOI] [PubMed] [Google Scholar]
- 9. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 10. Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30:303–71. [DOI] [PubMed] [Google Scholar]
- 11. Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin B et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr 2009;10:1–25. [DOI] [PubMed] [Google Scholar]
- 12. Silbiger JJ. Advances in rheumatic mitral stenosis: echocardiographic, pathophysiologic, and hemodynamic considerartions. J Am Soc Echocardiogr 2021;34:709–22.e1. [DOI] [PubMed] [Google Scholar]
- 13. Izumi C, Eishi K, Ashihara K, Arita T, Otsuji Y, Kunihara T et al. JCS/JSCS/JATS/JSVS 2020 guidelines on the management of valvular heart disease. Circ J 2020;84:2037–119. [DOI] [PubMed] [Google Scholar]
- 14. Mohan JC, Patel AR, Passey R, Gupta D, Kumar M, Arora R et al. Is the mitral valve area flow-dependent in mitral stenosis? A dobutamine stress echocardiographic study. J Am Coll Cardiol 2002;40:1809–15. [DOI] [PubMed] [Google Scholar]
- 15. Abramowitz Y, Jilaihawi H, Chakravarty T, Mack MJ, Makkar RR. Mitral annulus calcification J Am Coll Cardiol 2015;66:1934–41 [DOI] [PubMed] [Google Scholar]
- 16. Guerrero M, Wang DD, Pursnani A, Eleid M, Khalique O, Urena M et al. A cardiac computed tomography based score to categorize mitral annular calcification severity and predict valve embolization. JACC Cardiovasc Imaging 2020;13:1945–57. [DOI] [PubMed] [Google Scholar]
- 17. VARC-3 WRITING COMMITTEE; Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT et al. Valve Academic Research Consortium 3: updated endpoint definitions for aortic valve clinical research. Eur Heart J 2021;42:1825–57. [DOI] [PubMed] [Google Scholar]
- 18. Mejean S, Bouvier E, Bataille V, Seknadiji P, Fourchy D, Jean-Yves T et al. Mitral annular calcium and mitral stenosis determined by multidetector computed tomography in patients referred for aortic stenosis. Am J Cardiol 2016;118:1251–7. [DOI] [PubMed] [Google Scholar]
- 19. Kato N, Shibayama K, Omori N, Hoshina M, Makihara Y, Okumura H et al. Impact of transcatheter aortic valve replacement on hemodynamic status in patients with aortic stenosis and mitral stenosis: Doppler echocardiographic study. J Cardiol 2019;74:532–8. [DOI] [PubMed] [Google Scholar]
- 20. Sannino A, Potluri S, Pollock B, Filardo G, Gopal A, Stoler RC et al. Impact of mitral stenosis on survival in patients undergoing isolated transcatheter aortic valve implantation. Am J cardiol 2019;123:1314–20. [DOI] [PubMed] [Google Scholar]
- 21. Gentry JL III, Parikh PK, Alashi A, Gillinov A, Pettersson GB, Rodriguez LL et al. Characteristics and outcomes in a contemporary group of patients with suspected significant mitral stenosis undergoing treadmill stress echocardiograhpy. Circ Cardiovasc Imaging 2019;12:e009062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were provided by St. Marianna University Hospital. Data will be shared on request to the corresponding author with permission of St. Marianna University Hospital.