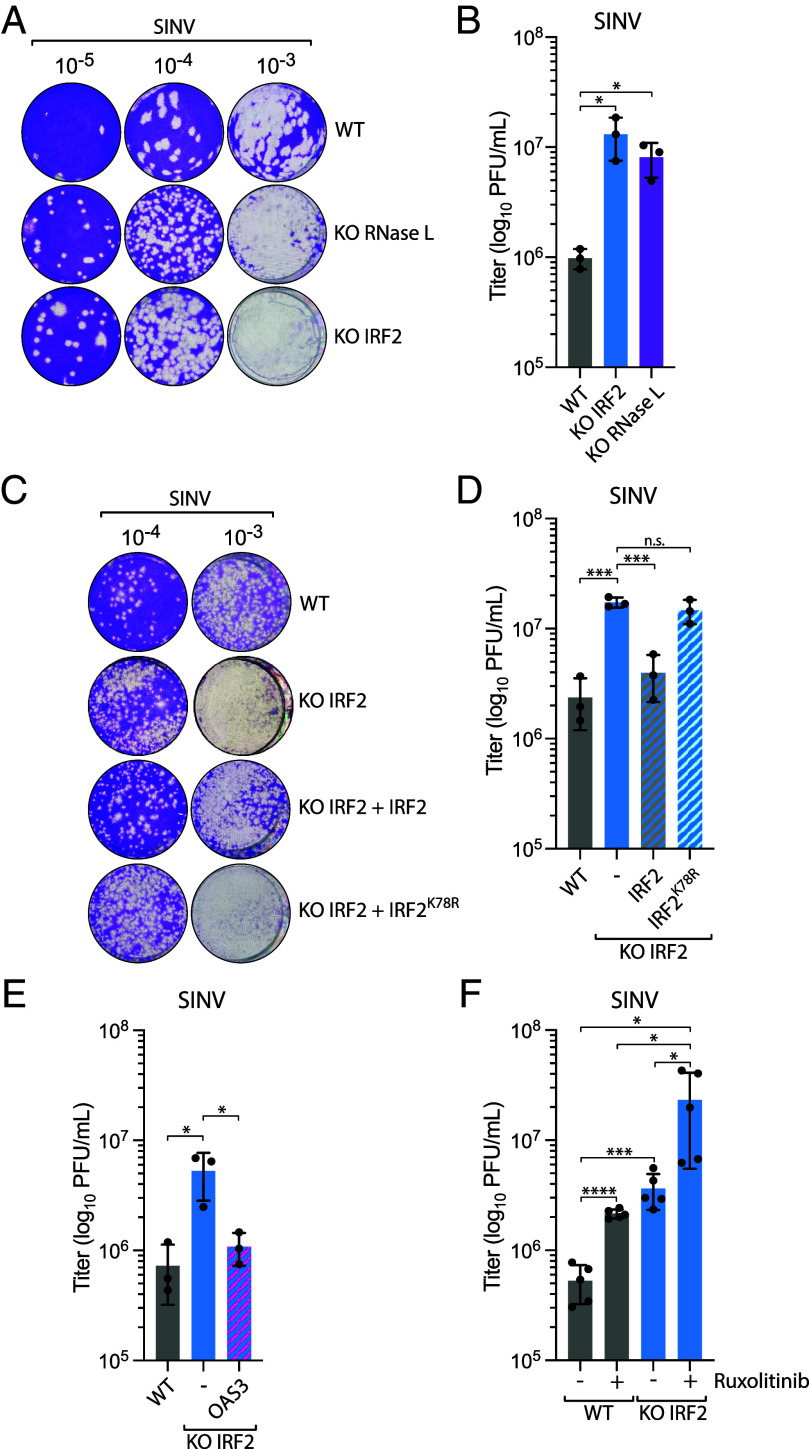
Fig. 5.
IRF2 suppresses SINV replication through the regulation of RNase L. (A) A549 WT, RNase L KO, or IRF2 KO cells were infected with SINV (MOI =10, 24 hpi), and the titer of infectious virus from the supernatant was determined by the plaque assay in Vero cells. Representative images of the plaque-forming assay were shown. (B) Quantification of viral titer (log10 PFU/mL) shown in A was determined by the plaque assay from three biological replicates. Mean values ± SD. *P < 0.05. (C) A549 WT or IRF2 KO cells complemented with the indicated construct were infected with SINV (MOI = 10, 24 hpi), and the titer of infectious virus from the supernatant was determined by the plaque assay in Vero cells. Representative images of the plaque-forming assay were shown. (D) Quantification of viral titer (log10 PFU/mL) shown in C was determined by the plaque assay from three biological replicates. Mean values ± SD. ***P < 0.001. (E) A549 WT or IRF2 KO cells complemented with OAS3 were infected with SINV (MOI = 10, 24 hpi), and the titer of infectious virus from the supernatant was determined by the plaque assay in Vero cells to determine the viral titer (log10 PFU/mL) from three biological replicates. Mean values ± SD. *P < 0.05. (F) A549 WT or IRF2 KO cells treated with ruxolitinib (2 μM) were infected with SINV (MOI = 10, 24 hpi), and the titer of infectious virus from the supernatant was determined by the plaque assay in Vero cells to determine the viral titer (log10 PFU/mL) from three biological replicates. Mean values ± SD. *P < 0.05; ***P < 0.001; ****P < 0.0001.