Abstract
A 59-year-old female being treated for multiple myeloma (MM) was referred to the Division of Cardiology due to edema and dyspnea. She developed dyspnea on exertion 2 months previously when carfilzomib, a second-generation selective proteasome inhibitor which was approved for treatment of relapsed and refractory MM, was introduced, and facial edema appeared thereafter. The electrocardiogram showed sinus rhythm with T-wave inversion on extensive leads and the chest X-ray showed cardiomegaly. Although cancer therapeutics-related cardiac dysfunction was assumed to be the complication, echocardiogram revealed no evidence of elevated left ventricular filling pressure whereas elevated tricuspid regurgitation velocity (3.2 m/s) with right ventricular systolic dysfunction suggested pre-capillary pulmonary hypertension (PH). Right heart catheterization demonstrated elevated mean pulmonary artery pressure (33 mmHg) along with high pulmonary vascular resistance (11.54 Wood Units) and normal pulmonary capillary wedge pressure (9 mmHg), confirming the echocardiographic findings. After ruling out other causes, PH associated with carfilzomib was diagnosed. Cessation of carfilzomib along with pulmonary vasodilator therapy led to improvement of symptoms and reduction of right heart size along with reduced estimated pulmonary systolic pressure 2 months later. Although carfilzomib-induced PH is rare, we need to consider its possibility when we find PH in patients receiving carfilzomib.
Learning objective
While adverse cardiovascular events are often found in patients with multiple myeloma (MM) after use of carfilzomib, the occurrence of pulmonary hypertension (PH) is reported to be rare. Because temporal association of echocardiographic findings to carfilzomib therapy plays a key role for the diagnosis of drug-associated PH, serial echocardiographic examinations should be performed when we start carfilzomib therapy in refractory MM patients.
Keywords: Carfilzomib, Pulmonary hypertension, Multiple myeloma, Echocardiography
Introduction
Proteasome inhibitors (PI) act as a cornerstone of multiple myeloma (MM) treatment regimens. Carfilzomib, a second-generation PI, has improved treatment of relapsed or refractory MM and has expanded the treatment options for these patients [1]. At the same time, use of carfilzomib has been reported to be associated with increased risk of cardiovascular adverse events including heart failure, myocardial ischemic events, or venous thromboembolic events [2,3]. Of these, prevalence of pulmonary hypertension (PH) was rare [4] and case reports showing adverse events related to carfilzomib-associated PH are limited. We herein report a case of carfilzomib-induced PH diagnosed in an adult refractory MM patient who developed PH and right ventricular (RV) dysfunction after carfilzomib use.
Case report
A 59-year-old female with a 2 year-history of treatment for IgG κ-type MM with daratumumab, bortezomib, and melphalan followed by daratumumab and lenalidomide became resistant to the medications. The regimen was then switched to carfilzomib/dexamethasone combination therapy. Transthoracic echocardiography (TTE) performed just before starting carfilzomib showed normal ventricular cavity sizes, wall thickness, valvular function, and estimated systolic pulmonary artery pressure (SPAP) (Fig. 1). One month after initiating carfilzomib, the patient began to experience transient palpitations lasting several minutes. By the end of the third course of treatment, symptoms of fatigue, exertional dyspnea, and facial edema emerged, and her attending physician consulted the cardiovascular division suspecting cancer therapeutics-related cardiac dysfunction (CTRCD).
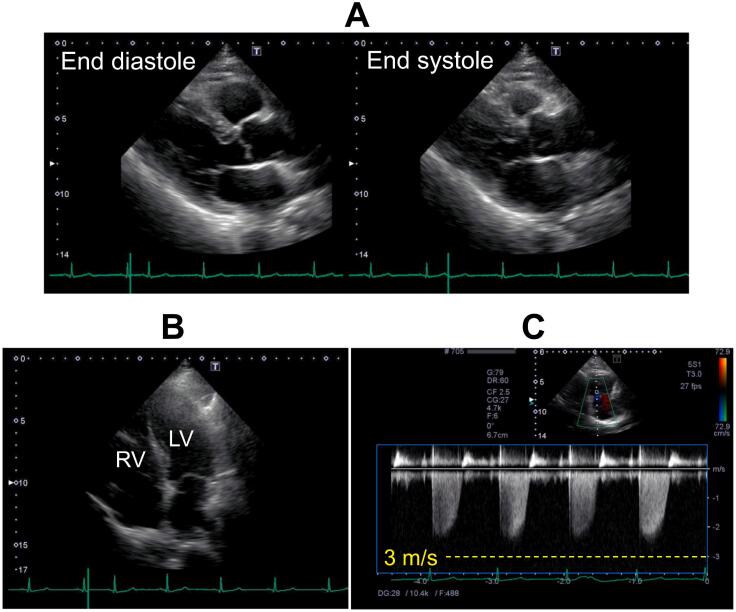
Fig. 1.
Transthoracic echocardiographic images before use of carfilzomib. Parasternal long-axis view at end diastole and end-systole (A), apical four-chamber view at end-diastole (B), and continuous Doppler waveforms of tricuspid velocity (C) are presented. The findings denied any geometric and functional abnormalities in left ventricle and right ventricle (A, B). Peak tricuspid regurgitation pressure gradient was 23 mmHg (C).
LV, left ventricle; RV, right ventricle.
On physical examination, she showed a heart rate of 92 bpm, blood pressure of 108/72 mmHg, respiratory rate of 30 breaths per minute, an oxygen saturation on pulse oximetry of 92 % on room air, absence of jugular venous distention, and significant bilateral lower limb edema. An electrocardiogram revealed T-wave inversions in leads V1-V6 and chest X-ray showed a cardiomegaly, both of which were not observed before the initiation of carfilzomib (Fig. 2A, B). Blood tests indicated normal levels of cardiac troponins and an elevated level of brain natriuretic peptide (BNP) (795 pg/mL). These findings led the physicians to diagnose heart failure due to CTRCD. However, TTE revealed markedly dilated RV with severe systolic dysfunction, severe tricuspid regurgitation (TR), and elevated peak TR velocity (3.2 m/s) along with enlarged inferior vena cava without respiratory variation while the left heart showed normal systolic function and denied findings of elevated filling pressure (E wave velocity: 32.6 cm/s, E/A: 0.6, E/e′: 4.5, left atrial volume index: 21.3 mL/m2), suggesting severe PH without left-sided heart failure (Fig. 3A-C). Subsequent right heart catheterization (RHC) confirmed pre-capillary PH with a mean pulmonary artery pressure (mPAP) of 33 mmHg, a mean pulmonary capillary wedge pressure of 9 mmHg, cardiac index (CI) of 1.39 L/min/m2, and pulmonary vascular resistance (PVR) of 11.54 Wood Units. After ruling out of other causes of PH such as acute pulmonary embolization, chronic thromboembolic PH, pre-existing lung disease, and connective tissue disease she was diagnosed with carfilzomib-associated PH based on the temporal association with carfilzomib initiation.
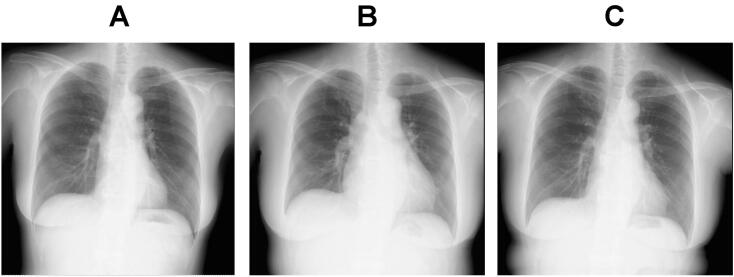
Fig. 2.
Chest X-rays before carfilzomib administration (A), on admission (B), and 2 months after discharge (C).
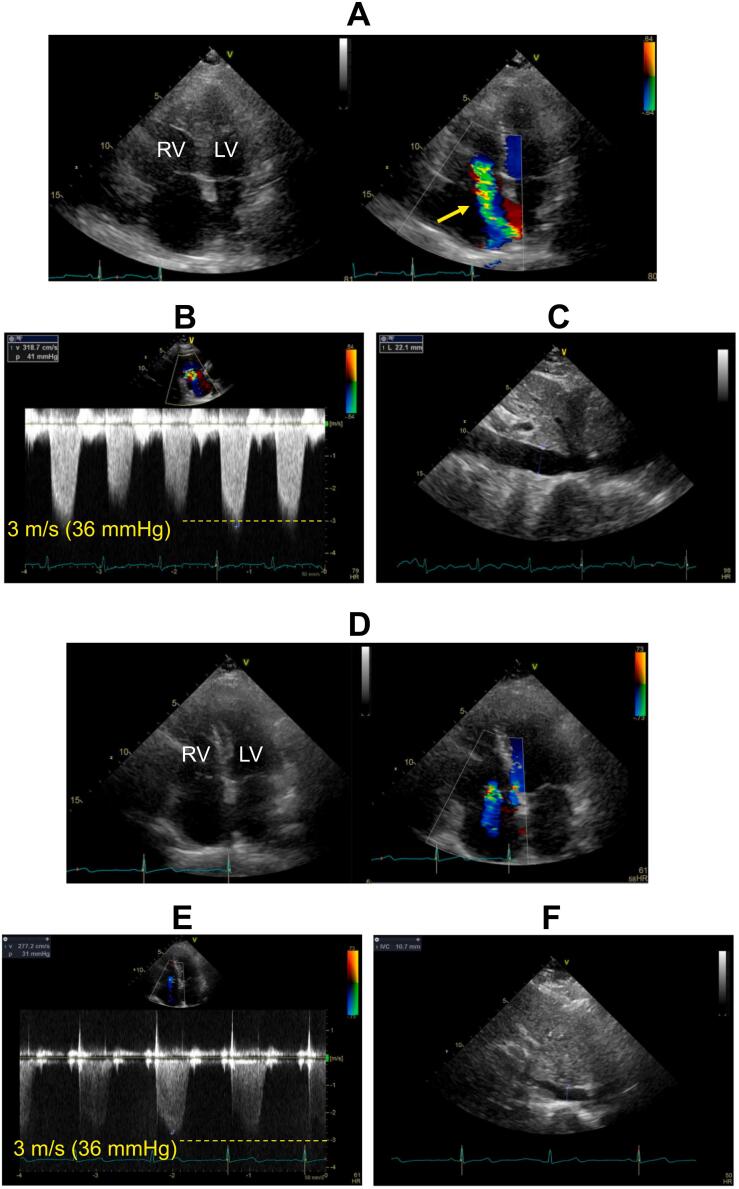
Fig. 3.
Transthoracic echocardiographic images during follow up. The images were obtained at discharge (A to C) and 2 months after discharge (D to F). (A) Two-dimensional and color Doppler images of apical four-chamber view at mid-systole showing markedly enlarged right ventricle and severe functional tricuspid regurgitation (arrow). (B) Subcostal long-axis view of the inferior vena cava (IVC) showing enlarged IVC, suggesting elevated right atrial pressure (RAP). (C) Tricuspid regurgitation (TR) velocity waveforms showed that peak systolic pressure gradient between right ventricle and right atrium was 41 mmHg. Accordingly, systolic pulmonary artery pressure (SPAP) was estimated as 56 mmHg from the formula: 41 (TR pressure gradient) + 15 (estimated RAP). (D) Two-dimensional and color Doppler images of apical four-chamber view at mid-systole showed shrinkage of the right ventricle as well as reduced tricuspid regurgitation. (E) Subcostal long-axis view of the IVC showing shrinkage of the IVC, suggesting normal RAP. (F) Continuous Doppler image of TR showed that peak TR pressure gradient was reduced to 31 mmHg. SPAP was thus estimated as 36 mmHg (31: TR pressure gradient +5: estimated RAP).
LV, left ventricle; RV, right ventricle.
To relieve the volume retention, intravenous diuretics were started and switched to oral administration. Carfilzomib was discontinued and pulmonary vasodilator therapy with phosphodiesterase-5 inhibitor and endothelin receptor antagonist along with prostaglandin derivatives was administered, although the third was not strongly recommended as a first-line therapy by the guidelines [5]. Fortunately, there was no need for positive inotropic agents because she denied any signs of hypoperfusion during the treatment. On the 16th day, repeat TTE showed reduction in RV size with improved wall motion, reduced TR, while elevated SPAP was similar to before the treatment. RHC on the 20th day revealed slightly decreased mPAP (30 mmHg), significantly increased CI (2.9 L/min/m2), and reduced PVR (3.76 Wood Units), suggesting an improved hemodynamics after the pulmonary vasodilator therapy. Symptom relief was achieved and she was discharged on the 23rd day. Two months after the discharge, a follow-up chest X-ray showed a shrinkage of cardiac silhouette (Fig. 2C) and blood test showed a reduction in plasma BNP level to 55 pg/mL. Follow-up TTE demonstrated further reduction of RV size, normalization of RV systolic function, and reduced SPAP (from 56 mmHg to 36 mmHg) (Fig. 3D-F). Alternative therapy using pomalidomide and bortezomib for MM was restarted and she is now well without cardiac symptoms.
Discussion
Although the frequency of PH is reported to be more prevalent in patients with MM than in the general population due to coincidence of left heart failure, venous thromboembolism, pulmonary vasculopathy, high-output status caused by anemia, and hyper viscosity status [6], treatment-associated PH in MM is relatively rare [6]. PIs induce cellular apoptosis via endoplasmic reticulum stress in MM cells in which degrading of the misfolding proteins largely depends on the ubiquinone-proteasome system [7]. Carfilzomib, a second-generation PI, has improved the treatment of relapsed and refractory MM, enhancing therapeutic strategies beyond conventional treatments. At the same time, adverse cardiac events have been frequently reported at incidence rates of 9.5 % to 22 % [2,3], which is thought to be due to a cardiotoxicity caused by decrease in proteasome activity in the myocardium [2]. Of these, development of PH is reported to be rare. One report based on a US Medicare-linked database showed that PH was recorded in only 1 % of the MM patients treated with carfilzomib [4]. Nevertheless, precise clinical features of carfilzomib-associated PH remain unclear because of the limited number of case reports. As far as we know, there have been three reports showing clinical features of MM patients suffering from carfilzomib-associated PH [[8], [9], [10]], and ours is the fourth report. A common feature among these cases was the appearance of symptoms after 3 to 4 cycles of carfilzomib, or after 2 to 4 months of treatment and the diagnoses were based on changes in echocardiographic findings from those before medication. While early stage of the PH was inferred to be reversible because of remission after discontinuation of carfilzomib [[8], [9], [10]], severe PH needed specific treatment for PH as in the present case [10].
There were two important matters in the clinical course of the present case. First, the initial manifestation of the patient such as exertional dyspnea, lower limb edema, cardiomegaly on chest X-ray, and elevated plasma BNP levels after initiation of carfilzomib made the physician suspect the occurrence of CTRCD, whereas the true cause was PH. We need to keep the development of PH in mind when managing patients treated with carfilzomib. Second, the normal echocardiographic findings before the initiation of carfilzomib along with typical PH findings without left ventricular systolic and diastolic dysfunction after occurrence of symptoms played an important role in the diagnosis of carfilzomib-associated PH. The present case thus underlines the importance of serial echocardiographic examinations in patients undergoing carfilzomib therapy. In conclusion, we present a rare MM case of PH associated with carfilzomib, in whom reduction of pulmonary pressure was achieved by discontinuation of carfilzomib along with pulmonary vasodilator therapy.
Patient permission/consent statement
Informed consent was obtained from the patient for the publication of the case and accompanying images.
Funding statement
Not applicable.
Declaration of competing interest
The authors have nothing to be disclosed regarding this case report.
Acknowledgment
The authors thank Drs Mirei Nabuchi-Kawasaki, Keigo Misonou, Taro Tsuzuki, Atsunari Utsugi, Daigo Nagahara, and Mitsugu Hirokami for their assistance in the patient's treatment. We also appreciate the sonographers of the cardiovascular ultrasound center in Teine Keijinkai Hospital for acquisition of the echocardiographic images.
References
- 1.Muchtar E., Gertz M.A., Magen H. A practical review on carfilzomib in multiple myeloma. Eur J Haematol. 2016;96:564–577. doi: 10.1111/ejh.12749. [DOI] [PubMed] [Google Scholar]
- 2.Shah C., Bishnoi R., Jain A., Bejjanki H., Xiong S., Wang Y., Zou F., Moreb J.S. Cardiotoxicity associated with carfilzomib: systematic review and meta-analysis. Leuk Lymphoma. 2018;59:2557–2569. doi: 10.1080/10428194.2018.1437269. [DOI] [PubMed] [Google Scholar]
- 3.Patel V.G., Cornell R.F. Cardiovascular complications associated with multiple myeloma therapies: incidence, pathophysiology, and management. Curr Oncol Rep. 2019;21:29. doi: 10.1007/s11912-019-0784-4. [DOI] [PubMed] [Google Scholar]
- 4.Fakhri B., Fiala M.A., Shah N., Vij R., Wildes T.M. Measuring cardiopulmonary complications of carfilzomib treatment and associated risk factors using the SEER-Medicare database. Cancer. 2020;126:808–813. doi: 10.1002/cncr.32601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukuda K., Date H., Doi S., Fukumoto Y., Fukushima N., Hatano M., Ito H., Kuwana M., Matsubara H., Momomura S.I., Nishimura M., Ogino H., Satoh T., Shimokawa H., Yamauchi-Takihara K., et al. Guidelines for the treatment of pulmonary hypertension (JCS 2017/JPCPHS 2017) Circ J. 2019;83:842–945. doi: 10.1253/circj.CJ-66-0158. [DOI] [PubMed] [Google Scholar]
- 6.Maddipati V., Sankhyan P., Goswami D.P., Mahajan A. Pulmonary hypertension in patients with multiple myeloma: a comprehensive review. Pulm Circ. 2023;13 doi: 10.1002/pul2.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitsiades C.S. Therapeutic landscape of carfilzomib and other modulators of the ubiquitin-proteasome pathway. J Clin Oncol. 2015;33:782–785. doi: 10.1200/JCO.2014.55.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGregor P.C., Boosalis V., Aragam J. Carfilzomib-induced pulmonary hypertension with associated right ventricular dysfunction: a case report. SAGE Open Med Case Rep. 2021;9 doi: 10.1177/2050313X21994031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathur P., Thanendrarajan S., Lopez-Candales A. Severe right-sided heart failure and pulmonary hypertension with carfilzomib treatment in multiple myeloma. Heart Views. 2020;21:296–299. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_107_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J.Z., Buckstaff T., Narezkina A., Fernandes T.M. Carfilzomib-associated pulmonary arterial hypertension in multiple myeloma. Pulm Circ. 2021;11 doi: 10.1177/20458940211049300. [DOI] [PMC free article] [PubMed] [Google Scholar]