Abstract
BACKGROUND
The combination of transarterial chemoembolization (TACE), lenvatinib, and programmed cell death 1 (PD-1) inhibitor has been widely used in the treatment of advanced hepatocellular carcinoma (HCC) and has achieved promising results. However, there are few studies comparing whether drug-eluting beads TACE (D-TACE) can bring more survival benefits to patients with large HCC compared to conventional TACE (C-TACE) in this triplet therapy.
AIM
To compare the efficacy and adverse events (AEs) of triple therapy comprising D-TACE, PD-1 inhibitors, and lenvatinib (D-TACE-P-L) and C-TACE, PD-1 inhibitors, and lenvatinib (C-TACE-P-L) in patients with large HCC (maximum diameter ≥ 5 cm), and analyze the prognostic factors.
METHODS
Following a comprehensive review of our hospital’s medical records, this retrospective study included 104 patients: 50 received D-TACE-P-L, and 54 received C-TACE-P-L. We employed Kaplan-Meier estimation to assess the median progression-free survival (PFS) between the two groups, utilized Cox multivariate regression analysis to identify prognostic factors, and applied the χ2 test to evaluate AEs.
RESULTS
The objective response rate (ORR) and median PFS were significantly higher in the D-TACE-P-L group compared to the C-TACE-P-L group (ORR: 66.0% vs 44.4%, P = 0.027; median PFS: 6.8 months vs 5.0 months, P = 0.041). Cox regression analysis identified treatment option, portal vein tumor thrombus, and hepatic vein invasion as protective factors for PFS. AEs were comparable between the two groups.
CONCLUSION
D-TACE-P-L may have significantly better PFS and ORR for large HCC, while exhibiting similar AEs to C-TACE-P-L.
Keywords: Large hepatocellular carcinoma, Conventional transarterial chemoembolization, Drug-eluting beads transarterial chemoembolization, Programmed cell death 1 inhibitor, Lenvatinib
Core Tip: A retrospective analysis encompassing 104 patients diagnosed with large hepatocellular carcinoma (≥ 5 cm), focused on comparing the efficacy and safety of two treatment modalities, which were the triple combination therapy of drug-eluting beads transarterial chemoembolization (D-TACE), programmed cell death 1 inhibitor, and lenvatinib (D-TACE-P-L) and the triple therapy consisting of conventional TACE, programmed cell death protein 1 inhibitor, and lenvatinib. Progression-free survival, tumor response, and adverse events were compared between the two groups, and the findings revealed that D-TACE-P-L demonstrated significantly superior median progression-free survival and objective response rate, while maintaining comparable toxicity profiles. Based on these outcomes, this study proposed that the D-TACE-P-L therapy served as a preferential treatment option for individuals suffering from large hepatocellular carcinoma.
INTRODUCTION
Hepatocellular carcinoma (HCC) ranks as the sixth most prevalent cancer worldwide and is associated with a poor prognosis. Notably, Chinese patients constitute approximately half of the global HCC caseload[1,2]. Due to insidious onset and rapid progression, large lesions, defined as having a maximum diameter exceeding 5 cm, are frequently diagnosed at an advanced stage, rendering them ineligible for surgical resection[3].
Currently, transarterial chemoembolization (TACE) is a widely adopted technique for managing unresectable HCC (uHCC)[4]. However, conventional TACE (C-TACE), which employs lipiodol mixed with chemotherapeutic agents, demonstrates limited efficacy in treating large HCC lesions. The objective response rate (ORR) ranges from 16% to 29%, while overall survival (OS) is restricted to between 6.5 and 9.9 months[5-7]. In contrast, drug-eluting beads TACE (D-TACE) offers sustained drug release alongside persistent embolization, potentially enhancing treatment outcomes for HCC[8]. For large or massive HCC tumors, D-TACE has been shown to achieve higher ORR rates, longer progression-free survival (PFS), and fewer adverse reactions compared to C-TACE[9,10]. Nevertheless, monotherapy with TACE remains inadequate regarding effectiveness; local recurrence rates at three months, six months, and one year are reported to be 18.6%, 33.4%, and 61.8%, respectively[11]. The TACE procedure induces a hypoxic environment within residual tumor tissue that stimulates increased production of vascular endothelial growth factor, leading to neovascularization - a significant contributor to tumor recurrence post-TACE. Furthermore, TACE does not effectively counteract immune evasion by tumor cells.
In recent years, substantial advancements have been made in systemic therapies for HCC encompassing tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors. TKIs have demonstrated efficacy in inhibiting tumor vessel proliferation while improving median OS among patients with HCC; thus, sorafenib and lenvatinib are recommended as first-line TKIs for uHCC management[12,13]. Immunotherapy has the potential to effectively inhibit the immune evasion mechanisms employed by tumor cells. Although immunotherapy alone has not exhibited superior efficacy relative to traditional treatments for HCC on its own merit, numerous studies indicate that combining TKIs with programmed cell death 1 (PD-1) inhibitors yields improved outcomes for patients suffering from this malignancy[14-18].
Therapies that inhibit angiogenesis, such as antibodies directed against vascular endothelial growth factor or TKIs, may postpone the revascularization and recurrence of tumors following TACE. PD-1 inhibitors can limit immune evasion, thereby enhancing the immune response to kill tumor cells. Due to their synergistic effect, combined therapy with TACE, PD-1 inhibitors, and lenvatinib has been proven to have better efficacy in treating uHCC[13,19]. However, the efficacy of combined therapy with C-TACE, PD-1 inhibitors, and lenvatinib (C-TACE-P-L) for treating large HCC remains unsatisfactory. D-TACE combined with PD-1 inhibitor and lenvatinib (D-TACE-P-L) may be better than C-TACE-P-L. Current available clinical studies on the utilization of D-TACE-P-L for large HCC are scarce, reflecting the need for further investigations and clinical trials to fully assess its potential benefits and risks in this specific patient population. Consequently, this retrospective study aimed to evaluate and compare the efficacy and safety of these two therapies in patients with large HCC.
MATERIALS AND METHODS
Patient criteria
Our research strictly adhered to the ethical principles outlined in the Declaration of Helsinki. The ethics committee of Ningbo No. 2 Hospital conducted a thorough review of our study and granted its approval. Since this study was conducted retrospectively, the ethics committee waived the necessity for informed consent. Furthermore, data were collected and analyzed anonymously to ensure participant privacy and confidentiality. Data were obtained from a cohort of patients with large HCC who received either D-TACE-P-L or C-TACE-P-L between May 1, 2019, and December 1, 2022. The inclusion criteria for our study included: (1) Age between 18-75 years; (2) Diagnosis of HCC confirmed by pathological examination; (3) Treatment with either D-TACE-P-L or C-TACE-P-L; and (4) Presence of at least one measurable lesion that exhibited arterial enhancement, with the largest lesion exceeding 5 cm in diameter. Patients meeting any of the following exclusion criteria were excluded: (1) Eastern Cooperative Oncology Group Performance Status score > 1; (2) Receipt of other anticancer treatments; (3) Concurrent Child-Pugh grade C status; (4) Presence of other malignancies; or (5) Incomplete medical records or information. Magnetic resonance imaging (MRI), or contrast-enhanced computed tomography (CT), was performed within one week prior to initial treatment, along with all necessary laboratory tests completed within three days.
Treatment
All patients received intravenous injections of PD-1 inhibitors, including tislelizumab (200 mg), sintilimab (200 mg), toripalimab (240 mg), and camrelizumab (200 mg) every three weeks. Additionally, they were administered lenvatinib orally at a standard dose of 8 mg for those weighing less than 60 kg or 12 mg for those weighing 60 kg or more, or an individualized dose as appropriate. Furthermore, TACE procedures were performed every one to two months if enhanced CT or MRI indicated significant arterial blood supply to the tumor; PD-1 inhibitors and lenvatinib were withheld three days prior to and following TACE. Digital subtraction angiography was utilized to identify arteries supplying blood to the tumor during TACE. Subsequently, a microcatheter was inserted into these arteries based on patient preference for either D-TACE or C-TACE. Drug-eluting beads or iodized oil containing doxorubicin (20 mg in C-TACE and 50 mg in D-TACE) were then slowly injected through the microcatheters for embolization. The efficacy of embolization was assessed via digital subtraction angiography, with the procedure concluded upon achieving satisfactory results.
Evaluation criteria
According to the mRECIST criteria, lesions were classified as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD)[20]. The primary objectives were to assess the median PFS, ORR, disease control rate (DCR), and prognostic factors of PFS. The ORR was defined as the incidence of CR and PR. The DCR was defined as the incidence of CR, PR, or SD. PFS was defined as the duration from the first TACE session to the occurrence of PD, death, or the last day of follow-up. Additionally, we aimed to evaluate adverse events (AEs) as secondary outcomes. AEs were evaluated and graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (version 4.0).
Follow-up
Patients were monitored at intervals of 1 to 3 months following their initial TACE. During each follow-up visit, a comprehensive evaluation was performed, which included hematological and biochemical tests as well as contrast-enhanced CT or MRI scans. If the lesion was classified as PD or if the patient could not tolerate treatment, the original therapy was discontinued, and alternative appropriate treatment options were considered for these patients. The follow-up endpoint was established as May 1, 2024.
Statistical analyses
Categorical variables are presented as percentages and analyzed using the χ2 test. Continuous variables are reported as means ± standard deviations and compared with the Student’s t-test. The median PFS between the two groups was assessed using Kaplan-Meier estimation. We assessed clinical characteristics through Cox univariate analysis. The items that exhibited statistical significance (P < 0.1) were assessed again using Cox multivariate regression to uncover prognostic factors of PFS (P < 0.05). Other statistically significant differences were defined as those for which P < 0.05. All the statistical analyses were performed using SPSS Statistics version 24.3.
RESULTS
Patient characteristics
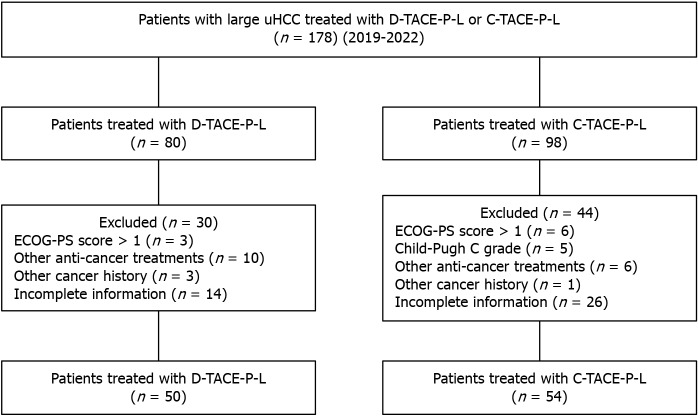
After reviewing the medical records of our hospital, we identified 178 patients with large HCC who received either D-TACE-P-L or C-TACE-P-L, meeting the inclusion criteria. A total of 74 patients were excluded based on the predefined exclusion criteria, as illustrated in Figure 1. Ultimately, our study included 104 patients: 50 in the D-TACE-P-L group and 54 in the C-TACE-P-L group. There were four categories of PD-1 inhibitors utilized: Tislelizumab for 14 patients (13.5%), sintilimab for 17 patients (16.3%), camrelizumab for 45 patients (43.3%), and toripalimab for 17 patients (26.9%). No statistically significant differences were observed in baseline characteristics (Table 1).
Figure 1.
Selection criteria. uHCC: Unresectable hepatocellular carcinoma; C-TACE-P-L: Conventional transarterial chemoembolization combined with lenvatinib plus programmed cell death 1 inhibitor; D-TACE-P-L: Drug-eluting beads transarterial chemoembolization combined with lenvatinib plus programmed cell death 1 inhibitor; ECOG-PS: Eastern Cooperative Oncology Group Performance Status.
Table 1.
Baseline characteristics of the patients
|
Characteristic
|
D-TACE-P-L group (n = 50)
|
C-TACE-P-L group (n = 54)
|
P value
|
| Sex, n (%) | 0.564 | ||
| Male | 42 (84.0) | 43 (79.6) | |
| Female | 8 (16.0) | 11 (20.4) | |
| Age (years), mean ± SD | 60.8 ± 9.2 | 62.2 ± 9.4 | 0.443 |
| ECOG PS, n (%) | 0.252 | ||
| 0 | 14 (28.0) | 10 (18.5) | |
| 1 | 36 (72.0) | 44 (81.5) | |
| Child-Pugh class, n (%) | 0.561 | ||
| A | 26 (52.0) | 25 (46.3) | |
| B | 24 (48.0) | 29 (53.7) | |
| BCLC, n (%) | 0.556 | ||
| B | 14 (28.0) | 18 (33.3) | |
| C | 36 (72.0) | 36 (66.7) | |
| AFP, n (%) | 0.727 | ||
| ≤ 400 ng/mL | 23 (46.0) | 23 (42.6) | |
| > 400 ng/mL | 27 (54.0) | 31 (57.4) | |
| Number of tumors, n (%) | 0.392 | ||
| ≤ 3 | 31 (62) | 29 (53.7) | |
| > 3 | 19 (38) | 25 (46.3) | |
| Largest tumor size (mm), mean ± SD | 96.3 ± 27.7 | 91.0 ± 36.7 | 0.324 |
| PVTT, n (%) | 0.873 | ||
| No | 23 (46.0) | 24 (44.4) | |
| Yes | 27 (54.0) | 30 (55.6) | |
| Hepatic vein invasion, n (%) | 0.656 | ||
| No | 38 (76.0) | 43 (20.4) | |
| Yes | 12 (24.0) | 11 (79.6) | |
| Extrahepatic metastasis, n (%) | 0.661 | ||
| No | 41 (82.0) | 46 (85.2) | |
| Yes | 9 (18.0) | 8 (14.8) | |
| Number of TACE, mean ± SD | 2.46 ± 1.0 | 2.56 ± 1.1 | 0.640 |
C-TACE-P-L: Conventional transarterial chemoembolization combined with lenvatinib plus programmed cell death 1 inhibitor; D-TACE-P-L: Drug-eluting beads transarterial chemoembolization combined with lenvatinib plus programmed cell death 1 inhibitor; ECOG PS: Eastern Cooperative Oncology Group Performance Status; BCLC: Barcelona Clinic Liver Cancer; AFP: Alpha-fetoprotein; PVTT: Portal vein tumor thrombus; TACE: Transarterial chemoembolization.
Tumor response
The tumor response and DCR were comparable between the two groups, with no significant differences observed (P = 0.113, P = 0.143; Table 2). However, a significant difference in ORR was noted between the groups (66.0% vs 44.4%, P = 0.027). In the D-TACE-P-L group, the percentages of CRs and PRs were 18.0% and 48.0%, whereas in the C-TACE-P-L group, these percentages were 7.4% and 37.0%. No significant differences were found among the various PD-1 inhibitor subgroups (P = 0.927; Table 3).
Table 2.
Tumor response, n (%)
|
Tumor response
|
D-TACE-P-L
|
C-TACE-P-L
|
P value
|
| CR | 9 (18.0) | 4 (7.4) | 0.113 |
| PR | 24 (48.0) | 20 (37.0) | |
| SD | 12 (24.0) | 19 (35.2) | |
| PD | 5 (10.0) | 11 (20.4) | |
| ORR (CR + PR) | 33 (66.0) | 24 (44.4) | 0.027 |
| DCR (CR + PR + SD) | 45 (90.0) | 43 (81.0) | 0.143 |
C-TACE-P-L: Conventional transarterial chemoembolization combined with lenvatinib plus programmed cell death 1 inhibitor; D-TACE-P-L: Drug-eluting beads transarterial chemoembolization combined with lenvatinib plus programmed cell death 1 inhibitor; CR: Complete response; PR: Partial response; SD: Stable disease; PD: Progressive disease; ORR: Objective response rate; DCR: Disease control rate.
Table 3.
Tumor response of different programmed cell death 1 inhibitor groups, n (%)
|
PD-1 inhibitor
|
CR
|
PR
|
SD
|
PD
|
P value
|
| Tislelizumab | 2 (14.3) | 5 (35.7) | 4 (28.6) | 3 (24.1) | 0.927 |
| Sintilimab | 1 (5.9) | 10 (58.8) | 5 (29.4) | 1 (5.9) | |
| Camrelizumab | 6 (13.3) | 17 (37.8) | 14 (31.1) | 8 (17.8) | |
| Toripalimab | 4 (14.3) | 12 (42.9) | 8 (28.5) | 4 (14.3) |
PD-1: Programmed cell death 1; CR: Complete response; PR: Partial response; SD: Stable disease; PD: Progressive disease.
PFS and analysis of its prognostic factors
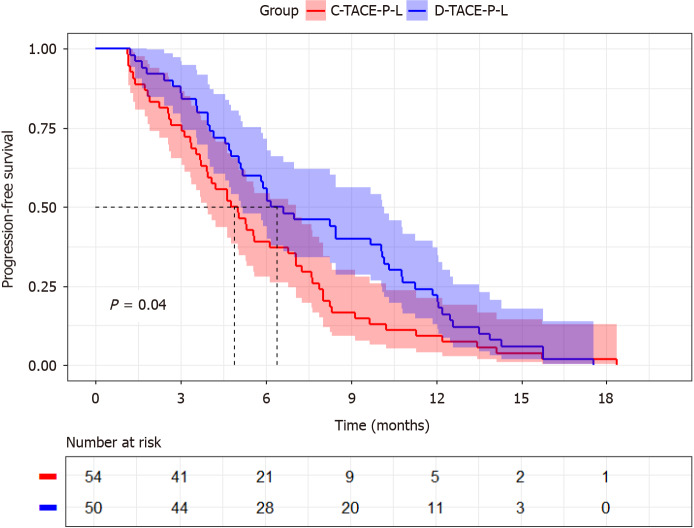
The D-TACE-P-L group exhibited a superior median PFS of 6.8 months [95% confidence interval (CI): 4.45-9.15] compared to that of the C-TACE-P-L group, which had a median PFS of 5.0 months (95%CI: 4.314-5.753). The hazard ratio (HR) was 1.422, with a 95%CI of 0.961-2.104, indicating a statistically significant difference (Figure 2, P = 0.041). Cox regression analysis (as detailed in Table 4) revealed the following prognostic factors for PFS: Portal vein tumor thrombus (PVTT) (No/Yes, HR = 1.670; 95%CI: 1.120-2.491; P = 0.012), hepatic vein invasion (No/Yes, HR = 1.807; 95%CI: 1.105-2.956; P = 0.018), and treatment option (D-TACE-P-L/C-D-TACE-P-L, HR = 1.536; 95%CI: 1.028-2.293; P = 0.036).
Figure 2.
Kaplan-Meier curves of progression-free survival. C-TACE-P-L: Conventional transarterial chemoembolization combined with lenvatinib plus programmed cell death 1 inhibitor; D-TACE-P-L: Drug-eluting beads transarterial chemoembolization combined with lenvatinib plus programmed cell death 1 inhibitor.
Table 4.
Univariate and multivariate analyses of risk factors for progression-free survival
| Factors |
Univariate analysis
|
Multivariate analysis
|
||||
|
HR
|
95%CI
|
P value
|
HR
|
95%CI
|
P value
|
|
| Sex | ||||||
| Male/female | 1.145 | 0.692-49 | 0.599 | |||
| Age (years) | 1.007 | 0.987-1.027 | 0.495 | |||
| ECOG PS | ||||||
| 0/1 | 1.246 | 0.781-1.988 | 0.357 | |||
| Child-Pugh class | ||||||
| A/B | 1.301 | 0.881-1.921 | 0.185 | |||
| AFP (ng/mL) | ||||||
| ≤ 400/> 400 | 1.215 | 0.821-1.798 | 0.329 | |||
| Number of tumors | ||||||
| ≤ 3/> 3 | 1.276 | 0.857-1.900 | 0.229 | |||
| Largest tumor size (mm) | 1.006 | 0.998-1.013 | 0.139 | |||
| PVTT | ||||||
| No/yes | 1.590 | 1.073-2.358 | 0.021 | 1.670 | 1.120-2.491 | 0.012 |
| Hepatic vein invasion | ||||||
| No/yes | 1.621 | 1.012-2.596 | 0.044 | 1.807 | 1.105-2.956 | 0.018 |
| Extrahepatic metastasis | ||||||
| No/yes | 1.778 | 1.038-3.044 | 0.036 | 1.554 | 0.900-2.686 | 0.114 |
| Treatment option | ||||||
| D-TACE-P-L/C-TACE-P-L | 1.422 | 0.961-2.104 | 0.078 | 1.536 | 1.028-2.293 | 0.036 |
C-TACE-P-L: Conventional transarterial chemoembolization combined with lenvatinib plus programmed cell death 1 inhibitor; D-TACE-P-L: Drug-eluting beads transarterial chemoembolization combined with lenvatinib plus programmed cell death 1 inhibitor; ECOG PS: Eastern Cooperative Oncology Group Performance Status; AFP: Alpha-fetoprotein; PVTT: Portal vein tumor thrombus; HR: Hazard ratio; CI: Confidence interval.
AEs
We summarized AEs in both groups (Table 5) and found that the most common AEs were fatigue (48.0% vs 37.0%), anorexia and nausea (52.0% vs 55.6%), rash (38% vs 46.3%), fever (92.0% vs 89.9%), and abdominal pain (68.0% vs 61.1%). The percentages of grade 3 AEs ranged from 0% to 12%, while no grade 4 or grade 5 AEs were observed. No statistically significant differences emerged in either the occurrence or severity of any AEs between the two groups, indicating comparable safety profiles overall; symptomatic treatment and dose reduction proved effective in mitigating these AEs.
Table 5.
Treatment-related adverse events in the two groups, n (%)
|
Adverse events
|
D-TACE-P-L (n = 50)
|
C-TACE-P-L (n = 54)
|
P value
|
||
|
Any grade
|
Grade 3
|
Any grade
|
Grade 3
|
||
| Diarrhea | 9 (18.0) | 2 (4.0) | 7 (13.0) | 1 (1.8) | 0.717 |
| Hand-foot syndrome | 12 (24.0) | 2 (4.0) | 15 (27.8) | 2 (3.7) | 0.882 |
| Hypertension | 13 (26.0) | 1 (2.0) | 14 (26.0) | 0 (0.0) | 0.572 |
| Fatigue | 24 (48.0) | 4 (8.0) | 20 (37.0) | 3 (5.6) | 0.522 |
| Anorexia and nausea | 26 (52.0) | 4 (8.0) | 30 (55.6) | 5 (9.3) | 0.928 |
| Rash | 19 (38.0) | 1 (2.0) | 25 (46.3) | 3 (5.6) | 0.518 |
| Thyroid dysfunction | 6 (12.0) | 0 (0.0) | 10 (18.5) | 1 (1.8) | 0.485 |
| Hyperbilirubinemia | 11 (22.0) | 2 (4.0) | 9 (16.7) | 1 (1.8) | 0.738 |
| Fever | 46 (92.0) | 6 (12.0) | 48 (89.9) | 3 (5.6) | 0.462 |
| Abdominal pain | 34 (68.0) | 6 (12.0) | 33 (61.1) | 2 (3.7) | 0.336 |
C-TACE-P-L: Conventional transarterial chemoembolization combined with lenvatinib plus programmed cell death 1 inhibitor; D-TACE-P-L: Drug-eluting beads transarterial chemoembolization combined with lenvatinib plus programmed cell death 1 inhibitor.
DISCUSSION
Iodized oil used in C-TACE consists of droplets of variable size that are prone to being washed away by blood flow, resulting in reperfusion of tumor blood vessels. In contrast, drug-eluting beads maintain a consistent size and can permanently occlude the artery supplying blood to the target tumor. Furthermore, D-TACE allows for sustained release of therapeutic agents directly into the tumor vasculature while minimizing systemic exposure. D-TACE has demonstrated efficacy and significantly reduces the incidence of AEs[21,22]. However, there remains no consensus on whether D-TACE is superior to C-TACE. Three randomized controlled trials reported varying outcomes[22-24]. Nevertheless, several studies have suggested that D-TACE may be more effective for treating large HCC[25-27]. For instance, a study conducted by Li et al[27] included patients with Barcelona Clinic Liver Cancer stage A/B and evaluated their response to therapy using mRECIST, revealing an ORR of 81.5% for D-TACE compared to 49.4% for C-TACE[27]. Additionally, research by Zhao et al[25] indicated median tumor diameters of 12.2 cm and 8.1 cm (P < 0.005) in the D-TACE group and C-TACE group, respectively; furthermore, both CRs and ORRs at one and three months were higher in the D-TACE group than those observed in the C-TACE group. These findings provide a foundation for our study aimed at verifying that D-TACE remains superior to C-TACE among patients with large uHCC when combined with lenvatinib and PD-1 inhibitors.
The combination therapy of TACE, lenvatinib, and PD-1 inhibitors is employed globally for the treatment of uHCC. PD-1 inhibitors function by disrupting signals that inhibit the immune system’s attack on tumors, thereby enhancing the immune response against cancerous cells[17,28]. The clinical efficacy of PD-1 inhibitors can be further augmented by reducing tumor blood supply and releasing tumor-specific antigens through TACE[29,30]. However, TACE induces a hypoxic microenvironment that may lead to tumor angiogenesis, recurrence, and metastasis. Fortunately, lenvatinib has been shown to inhibit angiogenesis and counteract tumor immunosuppressive mechanisms, thus improving clinical outcomes[31]. Due to the synergistic effects of this combination therapy involving TACE, lenvatinib, and PD-1 inhibitors, patients with uHCC demonstrate improved tumor responses and survival rates. Previous research has indicated that D-TACE enhances the infiltration of immune cells in tumor tissues, whereas C-TACE decreases it[32]. It is anticipated that incorporating immune checkpoint inhibitors will enhance the therapeutic efficacy of D-TACE and potentially extend OS and PFS. Our study found that D-TACE-P-L significantly improved median PFS from 5.0 months to 6.8 months compared with C-TACE-P-L (P = 0.041). However, due to the short follow-up time, more than 80% of the patients are still alive. It was not feasible to assess differences in OS adequately at this time point. Additionally, while there was a statistically significant difference in ORR, no such difference was observed in DCR. More than 90% of patients experienced PD during follow-up; therefore, this duration was sufficient for evaluating ORR and DCR effectively. A larger sample size may provide more robust validation regarding differences in ORR and DCR between groups.
Overall, our experimental results align well with theoretical predictions outlined previously. However similar studies are scarce within existing literature focused on triple therapy effectiveness for treating large uHCC where tumor diameter did not serve as an inclusion criterion or prognostic factor for PFS among treatment options like D-TACE vs C-TACE[33,34]. However, in one study, patients received either D-TACE or C-TACE in addition to camrelizumab[35]. The average tumor diameter (9.4 cm) in this study was significantly greater than that in other studies. The study suggested that D- TACE-C was superior to C-TACE-C in median PFS (10.0 months vs 3.0 months, P = 0.017). D-TACE-C shares similarities with D-TACE-P-L in mechanism, and enrolled participants with relatively large tumors. Thus, this similar result provides potentially relevant evidence for our outcomes. Nevertheless, further research remains essential.
Cox multivariate regression analysis identified PVTT, hepatic vein invasion, and treatment modality as independent prognostic factors for PFS. Numerous studies have corroborated that both PVTT and hepatic vein invasion serve as independent prognostic indicators for PFS, aligning with the findings of our study[33-35]. The presence of PVTT and hepatic vein invasion signifies a more advanced disease state, which is associated with poorer PFS outcomes. Furthermore, the treatment modality independently influenced prognosis, consistent with our hypothesis and reinforcing our conclusions.
Although D-TACE reduces the distribution of drugs to non-target regions, our study found no significant difference in the incidence of AEs. Similar findings have been reported in related studies[35,36]. In the investigation conducted by Ren et al[35], AEs included renal cell carcinoma embolization syndrome, rash, asthenia, anemia, and hypothyroidism; P values between the two groups were 0.111, 0.535, 0.484, 0.639, and 0.552, respectively, indicating no statistical significance.
However, several limitations must be acknowledged in this study. First, the retrospective design implies that treatment decisions were made by both physicians and patients, which may lead to selection bias. Additionally, the sample size was comparatively limited, highlighting the need for larger randomized controlled trials to provide more robust evidence. Finally, due to the short follow-up duration, we were unable to ascertain median OS, leaving uncertainty regarding whether D-TACE-P-L confers a superior OS compared to C-TACE-P-L.
CONCLUSION
D-TACE-P-L may have significantly better PFS and ORR for large HCC, while exhibiting similar AEs compared to C-TACE-P-L.
ACKNOWLEDGEMENTS
We are thankful for our families’ support, which has given us time to complete our research.
Footnotes
Institutional review board statement: The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Ningbo No. 2 Hospital (approval No: YJ-NBEY-KY-2024-004-01; date of approval: 2024-01-16).
Informed consent statement: The need for patient consent was waived due to the retrospective nature of the study which was approved by the Ethics Committee of Ningbo No. 2 Hospital.
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B, Grade B, Grade C
Novelty: Grade B, Grade B, Grade C
Creativity or Innovation: Grade B, Grade B, Grade C
Scientific Significance: Grade B, Grade B, Grade B
P-Reviewer: Fu A; Zerem E S-Editor: Wang JJ L-Editor: Webster JR P-Editor: Yuan YY
Contributor Information
Hui Yang, Department of Interventional Therapy, Ningbo No. 2 Hospital, Ningbo 315000, Zhejiang Province, China.
Guang-Ping Qiu, Department of Interventional Therapy, Ningbo No. 2 Hospital, Ningbo 315000, Zhejiang Province, China.
Jie Liu, Department of Interventional Therapy, Ningbo No. 2 Hospital, Ningbo 315000, Zhejiang Province, China.
Tie-Quan Yang, Department of Interventional Therapy, Ningbo No. 2 Hospital, Ningbo 315000, Zhejiang Province, China. younghc5@163.com.
Data sharing statement
The data underlying the findings of this study are accessible upon request to the corresponding author, owing to privacy and ethical considerations that preclude their public dissemination.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T, Nault JC, Neumann U, Ricke J, Sangro B, Schirmacher P, Verslype C, Zech CJ, Arnold D, Martinelli E ESMO Guidelines Committee. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv238–iv255. doi: 10.1093/annonc/mdy308. [DOI] [PubMed] [Google Scholar]
- 5.Yu SCH, Hui JW, Li L, Cho CC, Hui EP, Chan SL, Yeo WM. Comparison of Chemoembolization, Radioembolization, and Transarterial Ethanol Ablation for Huge Hepatocellular Carcinoma (≥ 10 cm) in Tumour Response and Long-Term Survival Outcome. Cardiovasc Intervent Radiol. 2022;45:172–181. doi: 10.1007/s00270-021-02777-6. [DOI] [PubMed] [Google Scholar]
- 6.Xue T, Le F, Chen R, Xie X, Zhang L, Ge N, Chen Y, Wang Y, Zhang B, Ye S, Ren Z. Transarterial chemoembolization for huge hepatocellular carcinoma with diameter over ten centimeters: a large cohort study. Med Oncol. 2015;32:64. doi: 10.1007/s12032-015-0504-3. [DOI] [PubMed] [Google Scholar]
- 7.Miyayama S, Kikuchi Y, Yoshida M, Yamashiro M, Sugimori N, Ikeda R, Okimura K, Sakuragawa N, Ueda T, Sanada T, Watanabe H, Notsumata K. Outcomes of conventional transarterial chemoembolization for hepatocellular carcinoma ≥10 cm. Hepatol Res. 2019;49:787–798. doi: 10.1111/hepr.13335. [DOI] [PubMed] [Google Scholar]
- 8.Song MJ, Chun HJ, Song DS, Kim HY, Yoo SH, Park CH, Bae SH, Choi JY, Chang UI, Yang JM, Lee HG, Yoon SK. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol. 2012;57:1244–1250. doi: 10.1016/j.jhep.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Huang J, Huang W, Zhan M, Guo Y, Liang L, Cai M, Lin L, He M, Lian H, Lu L, Zhu K. Drug-Eluting Bead Transarterial Chemoembolization Combined with FOLFOX-Based Hepatic Arterial Infusion Chemotherapy for Large or Huge Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2021;8:1445–1458. doi: 10.2147/JHC.S339379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayyub J, Dabhi KN, Gohil NV, Tanveer N, Hussein S, Pingili S, Makkena VK, Jaramillo AP, Awosusi BL, Nath TS. Evaluation of the Safety and Efficacy of Conventional Transarterial Chemoembolization (cTACE) and Drug-Eluting Bead (DEB)-TACE in the Management of Unresectable Hepatocellular Carcinoma: A Systematic Review. Cureus. 2023;15:e41943. doi: 10.7759/cureus.41943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kinugasa H, Nouso K, Takeuchi Y, Yasunaka T, Onishi H, Nakamura S, Shiraha H, Kuwaki K, Hagihara H, Ikeda F, Miyake Y, Takaki A, Yamamoto K. Risk factors for recurrence after transarterial chemoembolization for early-stage hepatocellular carcinoma. J Gastroenterol. 2012;47:421–426. doi: 10.1007/s00535-011-0492-9. [DOI] [PubMed] [Google Scholar]
- 12.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 13.Kimura T, Kato Y, Ozawa Y, Kodama K, Ito J, Ichikawa K, Yamada K, Hori Y, Tabata K, Takase K, Matsui J, Funahashi Y, Nomoto K. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018;109:3993–4002. doi: 10.1111/cas.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL KEYNOTE-240 investigators. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2020;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 15.Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, Kudo M, Harding JJ, Merle P, Rosmorduc O, Wyrwicz L, Schott E, Choo SP, Kelley RK, Sieghart W, Assenat E, Zaucha R, Furuse J, Abou-Alfa GK, El-Khoueiry AB, Melero I, Begic D, Chen G, Neely J, Wisniewski T, Tschaika M, Sangro B. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77–90. doi: 10.1016/S1470-2045(21)00604-5. [DOI] [PubMed] [Google Scholar]
- 16.Chiang HC, Lee YC, Chang TT, Lin YJ, Wu HT, Wang CT, Chen CY, Chen PJ, Hsieh MT, Lin SH, Chen SH, Chuang CH, Wu IC, Hong TC, Wu JS, Han MZ, Chen WT, Chiang CM, Hung KK, Kuo HY. Real-World Effectiveness of Sorafenib versus Lenvatinib Combined with PD-1 Inhibitors in Unresectable Hepatocellular Carcinoma. Cancers (Basel) 2023;15 doi: 10.3390/cancers15030854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rimassa L, Finn RS, Sangro B. Combination immunotherapy for hepatocellular carcinoma. J Hepatol. 2023;79:506–515. doi: 10.1016/j.jhep.2023.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Fong KY, Zhao JJ, Sultana R, Lee JJX, Lee SY, Chan SL, Yau T, Tai DWM, Sundar R, Too CW. First-Line Systemic Therapies for Advanced Hepatocellular Carcinoma: A Systematic Review and Patient-Level Network Meta-Analysis. Liver Cancer. 2023;12:7–18. doi: 10.1159/000526639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu XK, Yang LF, Chen YF, Chen ZW, Lu H, Shen XY, Chi MH, Wang L, Zhang H, Chen JF, Huang JY, Zeng YY, Yan ML, Zhang ZB. Transcatheter arterial chemoembolisation combined with lenvatinib plus camrelizumab as conversion therapy for unresectable hepatocellular carcinoma: a single-arm, multicentre, prospective study. EClinicalMedicine. 2024;67:102367. doi: 10.1016/j.eclinm.2023.102367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 21.Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S, Benhamou Y, Avajon Y, Gruenberger T, Pomoni M, Langenberger H, Schuchmann M, Dumortier J, Mueller C, Chevallier P, Lencioni R PRECISION V Investigators. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong K, Khwaja A, Liapi E, Torbenson MS, Georgiades CS, Geschwind JF. New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res. 2006;12:2563–2567. doi: 10.1158/1078-0432.CCR-05-2225. [DOI] [PubMed] [Google Scholar]
- 23.Sacco R, Bargellini I, Bertini M, Bozzi E, Romano A, Petruzzi P, Tumino E, Ginanni B, Federici G, Cioni R, Metrangolo S, Bertoni M, Bresci G, Parisi G, Altomare E, Capria A, Bartolozzi C. Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2011;22:1545–1552. doi: 10.1016/j.jvir.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Golfieri R, Giampalma E, Renzulli M, Cioni R, Bargellini I, Bartolozzi C, Breatta AD, Gandini G, Nani R, Gasparini D, Cucchetti A, Bolondi L, Trevisani F PRECISION ITALIA STUDY GROUP. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111:255–264. doi: 10.1038/bjc.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao C, Ma SPZCY. Comparison of treatment response, survival and safety between drug-eluting bead transarterial chemoembolization with CalliSpheres® microspheres versus conventional transarterial chemoembolization in treating hepatocellular carcinoma. J BUON. 2019;24:1150–1166. [PubMed] [Google Scholar]
- 26.Wu B, Zhou J, Ling G, Zhu D, Long Q. CalliSpheres drug-eluting beads versus lipiodol transarterial chemoembolization in the treatment of hepatocellular carcinoma: a short-term efficacy and safety study. World J Surg Oncol. 2018;16:69. doi: 10.1186/s12957-018-1368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Wang N, Shi C, Liu Q, Song J, Ye X. Short-term efficacy and safety of callispheres drug-loaded microsphere embolization in primary hepatocellular carcinoma. J Cancer Res Ther. 2021;17:733–739. doi: 10.4103/jcrt.JCRT_1848_20. [DOI] [PubMed] [Google Scholar]
- 28.Li B, Yan C, Zhu J, Chen X, Fu Q, Zhang H, Tong Z, Liu L, Zheng Y, Zhao P, Jiang W, Fang W. Anti-PD-1/PD-L1 Blockade Immunotherapy Employed in Treating Hepatitis B Virus Infection-Related Advanced Hepatocellular Carcinoma: A Literature Review. Front Immunol. 2020;11:1037. doi: 10.3389/fimmu.2020.01037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kudo M. A New Treatment Option for Intermediate-Stage Hepatocellular Carcinoma with High Tumor Burden: Initial Lenvatinib Therapy with Subsequent Selective TACE. Liver Cancer. 2019;8:299–311. doi: 10.1159/000502905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montasser A, Beaufrère A, Cauchy F, Bouattour M, Soubrane O, Albuquerque M, Paradis V. Transarterial chemoembolisation enhances programmed death-1 and programmed death-ligand 1 expression in hepatocellular carcinoma. Histopathology. 2021;79:36–46. doi: 10.1111/his.14317. [DOI] [PubMed] [Google Scholar]
- 31.Matsuki M, Hoshi T, Yamamoto Y, Ikemori-Kawada M, Minoshima Y, Funahashi Y, Matsui J. Lenvatinib inhibits angiogenesis and tumor fibroblast growth factor signaling pathways in human hepatocellular carcinoma models. Cancer Med. 2018;7:2641–2653. doi: 10.1002/cam4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doemel LA, Santana JG, Savic LJ, Gaupp FML, Borde T, Petukhova-Greenstein A, Kucukkaya AS, Schobert IT, Hamm CA, Gebauer B, Walsh JJ, Rexha I, Hyder F, Lin M, Madoff DC, Schlachter T, Chapiro J, Coman D. Comparison of metabolic and immunologic responses to transarterial chemoembolization with different chemoembolic regimens in a rabbit VX2 liver tumor model. Eur Radiol. 2022;32:2437–2447. doi: 10.1007/s00330-021-08337-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang H, Yang T, Qiu G, Liu J. Efficacy and Safety of TACE Combined with Lenvatinib and PD-(L)1 Inhibitor in the Treatment of Unresectable Hepatocellular Carcinoma: A Retrospective Study. J Hepatocell Carcinoma. 2023;10:1435–1443. doi: 10.2147/JHC.S423684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai M, Huang W, Huang J, Shi W, Guo Y, Liang L, Zhou J, Lin L, Cao B, Chen Y, Zhou J, Zhu K. Transarterial Chemoembolization Combined With Lenvatinib Plus PD-1 Inhibitor for Advanced Hepatocellular Carcinoma: A Retrospective Cohort Study. Front Immunol. 2022;13:848387. doi: 10.3389/fimmu.2022.848387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren Y, Guo Y, Chen L, Sun T, Zhang W, Sun B, Zhu L, Xiong F, Zheng C. Efficacy of Drug-Eluting Beads Transarterial Chemoembolization Plus Camrelizumab Compared With Conventional Transarterial Chemoembolization Plus Camrelizumab for Unresectable Hepatocellular Carcinoma. Cancer Control. 2022;29:10732748221076806. doi: 10.1177/10732748221076806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang W, Chen L, Cao Y, Sun B, Ren Y, Sun T, Zheng C. Efficacy of Drug-Eluting Beads Transarterial Chemoembolization Plus Apatinib Compared with Conventional Transarterial Chemoembolization Plus Apatinib in the Treatment of Unresectable Hepatocellular Carcinoma. Cancer Manag Res. 2021;13:5391–5402. doi: 10.2147/CMAR.S314762. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying the findings of this study are accessible upon request to the corresponding author, owing to privacy and ethical considerations that preclude their public dissemination.