Abstract
A 49-year-old woman with untreated severe mitral stenosis faced complex management challenges in a resource-limited setting. Initially presenting with abnormal uterine bleeding, her cardiac condition was discovered during a preoperative work-up for a planned hysterectomy. This case emphasizes the necessity for tailored and transdisciplinary management approaches.
Key Words: anticoagulation, atrial fibrillation, echocardiography, mitral valve, rheumatic heart disease, thrombus
Graphical Abstract
History of Presentation
A 49-year-old woman, gravida 6 para 6 from a disadvantaged community in Interior Sindh, Pakistan, was referred to the National Institute of Cardiovascular Diseases (NICVD) in Karachi. She had initially presented to a tertiary care hospital with pelvic discomfort and heavy abnormal uterine bleeding (AUB), where she was diagnosed with symptomatic uterine adenomyosis of 2.7 × 3.1 cm on the anterior wall. Per protocol, a hysterectomy was planned; however, during the patient’s preoperative work-up, an echocardiogram revealed severe mitral stenosis. Given the severity of the mitral stenosis , the anesthesia team did not grant general anesthesia fitness, necessitating the patient’s referral to NICVD for specialized cardiac care. At NICVD, she had a blood pressure of 118/74 mm Hg, a heart rate of 84 beats/min, a respiratory rate of 25 breaths/min, and an oxygen saturation of 98%. She exhibited a grade 3/6 diastolic murmur, which was most prominent at the apex and maintained its maximum intensity at expiration. Results of the patient’s laboratory tests are presented in Table 1.
Learning Objectives
-
•
To be able to formulate a transdisciplinary approach for managing patients with severe mitral stenosis with LAA thrombus in patients presenting with symptomatic adenomyosis.
-
•
To analyze the impact of severe mitral stenosis and LAA thrombus on the management of gynecologic conditions with high bleeding risk such as adenomyosis in resource-limited settings.
Table 1.
Laboratory Findings
| Result | Normal Range | |
|---|---|---|
| Biochemistry | ||
| Calcium, mg/dL | 9.2 | 8.4-10.2 |
| Creatinine, mg/dL | 0.53 | 0.55-1.02 |
| Magnesium, mg/dL | 1.84 | 1.6-2.6 |
| Phosphorus, mg/dL | 3.8 | 2.3-4.7 |
| Glucose, random blood glucose, mg/dL | 116 | Diabetes: ≥200 |
| Urea, mg/dL | 22 | 15-55 |
| Electrolytes | ||
| Serum sodium, mEq/L | 137 | 136-149 |
| Potassium, mEq/L | 4.1 | 3.5-5.0 |
| Serum chloride, mEq/L | 107 | 98-107 |
| Serum bicarbonate, mEq/L | 18 | 25-29 |
| Liver function tests | ||
| Bilirubin (total), mg/dL | 0.48 | 0.0-0.25 |
| Bilirubin (direct), mg/dL | 0.15 | 0.0-0.03 |
| GGT, U/L | 29 | 0-31 |
| SGPT (ALT), U/L | 24 | 0-37 |
| SGOT (AST), U/L | 64 | 0-37 |
| Blood coagulation | ||
| APTT, s | 20.2 | 21.9-29.7 |
| PT, s | 9.1 | 9.3-14.0 |
| INR | 0.86 | |
| Hematology | ||
| Hemoglobin, g/dL | 10.8 | 11.5-15.4 |
| Hematocrit, % | 32.6 | 35-47 |
| RBC, ×109/L | 3.44 | 4.10-5.10 |
ALT = alanine transaminase; APTT = activated partial thromboplastin time; AST = aspartate transaminase; GGT = gamma-glutamyl transferase; INR = international normalized ratio; PT = prothrombin time; RBC = red blood cells; SGOT = serum glutamic-oxaloacetic transaminase; SGPT = serum glutamic pyruvic transaminase.
Past Medical History
The patient's medical history is notable for complications associated with her last few pregnancies, which were all home births. She self-reported experiencing postpartum dyspnea, which suggests underlying cardiovascular issues that were not further investigated at the time. The lack of medical facilities and resources in her rural area contributed to the absence of a comprehensive work-up after her symptoms.
Differential Diagnosis
The initial concern for symptomatic severe mitral stenosis was heightened given the patient’s history of postpartum dyspnea. The presence of adenomyosis was already confirmed by the abdominal ultrasound.
Investigations
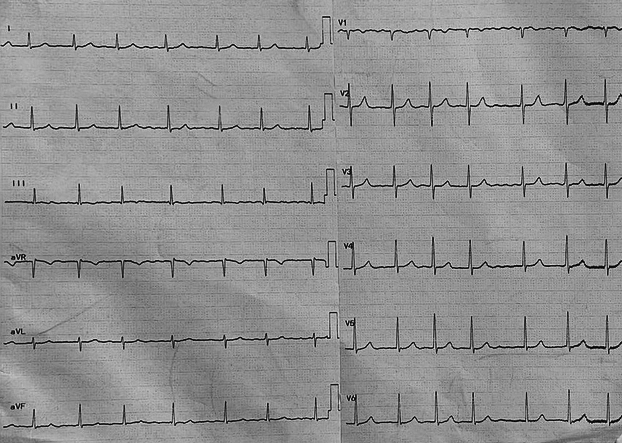
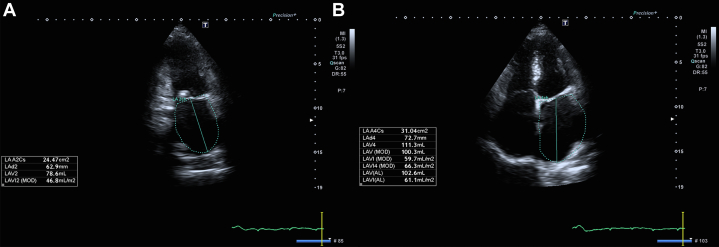
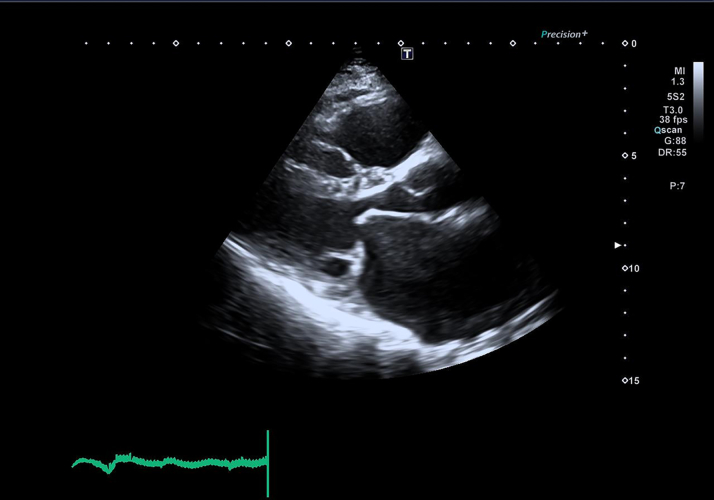
The electrocardiogram revealed atrial fibrillation (AF) (Figure 1), which is often associated with significant mitral stenosis. Transthoracic echocardiography further delineated the pathology, showing a severely dilated left atrium with a left atrial volume index (LAVI) of 49 mL/m2 (Figure 2) and a normal-sized left ventricle with normal systolic function, maintaining an ejection fraction of 55% (Table 2). The mitral valve apparatus was markedly abnormal with thickening and restricted mobility of posterior mitral leaflets and doming of anterior mitral leaflets, characteristic of rheumatic severe mitral stenosis (Figure 3); the mean gradient across the mitral valve was 15 mm Hg, and the mitral valve area according to planimetry was calculated at 0.79 cm2 (Figure 4). There was also evidence of mild mitral regurgitation and moderate to severe tricuspid regurgitation with an estimated pulmonary artery systolic pressure of 45 to 50 mm Hg.
Figure 1.
Electrocardiogram at Presentation
Electrocardiogram of patient on presentation showing atrial fibrillation with controlled heart rate.
Figure 2.
Left Atrial Volume Index on Transthoracic Echocardiogram
(A) Two-chamber view with tracing of the left atrium (LA). (B) Four-chamber view with tracing of the LA, showing a calculated LAVI of 61.1 mL/m2, indicating severe left atrial enlargement.
Table 2.
Echocardiography Findings
| Left atrium | Severely dilated (LAVI: 61.1 mL/m2) |
| Left ventricle | Normal size, ejection fraction: 55%; normal systolic function |
| Mitral valve | Thickened and restricted mobility; mean gradient: 15 mm Hg; valve area: 0.75 cm2; Wilkins score: 7/16 |
| Aortic valve | Mildly thickened, normal function |
| Tricuspid valve | Moderate to severe regurgitation |
| Pulmonary artery pressure | Elevated (estimated PASP: 45-50 mm Hg) |
| Mitral regurgitation | Mild |
| Aortic regurgitation | Mild |
| Flow abnormalities (color flow mapping) | Detected: mild mitral regurgitation, mild aortic regurgitation, moderate to severe tricuspid regurgitation |
LAVI = left atrial volume index; PASP = pulmonary artery systolic pressure.
Figure 3.
Characteristics of Mitral Stenosis on Transthoracic Echocardiogram
Parasternal long-axis view showing thickened mitral valve leaflets with doming of the anterior mitral leaflet, characteristic of rheumatic mitral stenosis.
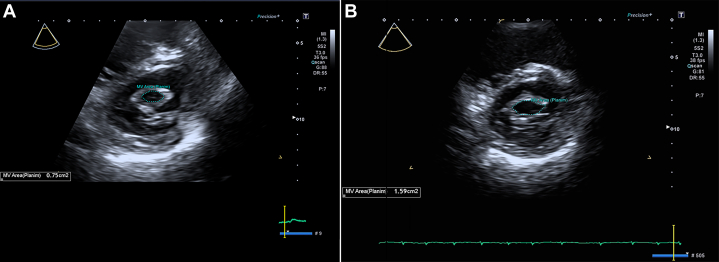
Figure 4.
Mitral Valve Area on Transthoracic Echocardiogram
(A) Pre-percutaneous mitral balloon valvotomy (PMBV) echocardiography showing a mitral valve area of 0.75 cm² by planimetry. (B) Post-PMBV echocardiography showing an increase in mitral valve area to 1.59 cm² by planimetry.
Transesophageal echocardiography confirmed these findings and revealed a severely dilated left atrium with contrast retention suggestive of thrombus formation within the LA appendage (LAA) (Supplemental Table 1, Video 1). The cardiac computed tomography scan provided additional support for the presence of a windsock-type LAA with an organized thrombus, a critical finding that would influence the therapeutic approach (Supplemental Table 2, Video 2).
The comprehensive diagnostic investigations concluded with a final picture of severe mitral stenosis with AF and pulmonary arterial hypertension, coupled with the presence of an LAA thrombus, thereby solidifying the diagnosis and guiding the subsequent management strategy for this patient.
Management
The patient presented with a challenging combination of severe mitral stenosis with AF, LAA thrombus, and severe pulmonary arterial hypertension.
The heavy AUB precluded the use of warfarin for LAA thrombus, while the LAA thrombus itself presented a barrier to percutaneous mitral balloon valvotomy (PMBV), which is often the first-line treatment for severe mitral stenosis. In addition, the planned hysterectomy to treat adenomyosis was deferred because of the high surgical risk posed by the severe mitral stenosis and AF.
Taking a transdisciplinary approach, 3 management steps were contemplated. The first step was PMBV, which aimed to urgently improve hemodynamics to a level that would permit the safe conduct of a hysterectomy. It was considered to provide symptomatic relief and potentially improve the patient’s candidacy for surgery by addressing the severe mitral stenosis. This approach was carefully executed, considering the patient’s severe mitral stenosis and the presence of LAA thrombus. To mitigate potential complications, several precautions were adhered to. Lower puncture in the interatrial septum was ensured to minimize the risk of inadvertent balloon migration into the LAA. In addition, heparin administration through the Mullins sheath was used, enhancing anticoagulation while minimizing the risk of thrombus formation. A nuanced technique was used to avoid balloon migration into the LAA, which could exacerbate the existing thrombus. By passing a Burman catheter into the left ventricle through the Mullins sheath and subsequently using a 3x5 Amplatz support wire (Boston Scientific), the risk of balloon migration was mitigated, allowing for precise placement either in the left ventricle or aorta.
Furthermore, it was crucial to prevent the PMBV wire from traversing too deeply into the LAA, especially given its typical mid-LA location. This precaution was essential to minimize the risk of dislodging or exacerbating the existing thrombus. After successful PMBV, the valve area increased from 0.75 cm2 to 1.59 cm2 (Supplemental Table 3).
Because the patient’s severe mitral stenosis was resolved, the gynecology consultant decided to proceed with total laparoscopic hysterectomy and bilateral salpingo-oophorectomy. The hysterectomy was uneventful. She was discharged on postoperative day 4 without any complications. On follow-up day 7, she reported NYHA functional class I symptoms and resolution of AUB.
The third step involved anticoagulation for the LAA thrombus. By resolving the source of the AUB, the patient was considered for anticoagulation therapy without the increased risk of hemorrhage, thereby managing the LAA thrombus more effectively. Anticoagulation was safely prescribed on postoperative day 7, and follow-up in 2 weeks was requested. Upon follow-up, the patient recovered well and did not report any thromboembolic events, AUB, or any other adverse outcomes.
We did not obtain institutional review board approval because as per the institutional policies, case reports do not require institutional review board approval. However, the patient's informed written consent was obtained.
Discussion
In low- and middle-income countries, the challenges of diagnosing mitral stenosis often result in delayed detection and poor prognosis for patients. Mitral stenosis, frequently a consequence of rheumatic heart disease, remains significantly underdiagnosed due to the reliance on echocardiography for definitive diagnosis.1 The delayed or missed diagnosis of mitral stenosis can lead to advanced valvular disease and potentially severe outcomes, including congestive heart failure, which complicates the management and reduces the likelihood of successful interventions.2 This issue is exemplified in the case report, in which a patient with symptomatic uterine adenomyosis was only diagnosed with severe mitral stenosis during preoperative assessments for a planned hysterectomy. This late discovery highlights the potential for significant cardiac conditions to remain hidden until patients undergo unrelated medical evaluations.3
In patients with untreated mitral stenosis, the risk of developing AF is notably high, which in turn significantly increases the risk of systemic embolization due to thrombus formation in the left atrium. Epidemiologically, the global prevalence of AF in patients with rheumatic heart disease is estimated at 32.8%.4 Among these patients, LA thrombi are a common complication, found in approximately 24% of those with severe mitral stenosis and concurrent AF.5 These thrombi significantly heighten the risk of embolic events, underscoring the critical need for timely diagnosis and intervention.6
In the case presented, the patient with severe mitral stenosis was concurrently diagnosed with symptomatic uterine adenomyosis, AF, and a windsock-type LAA-organized thrombus. According to the latest guidelines by the American College of Cardiology/American Heart Association, the presence of an LA thrombus in patients with severe mitral stenosis and AF necessitates immediate initiation of anticoagulation therapy, followed by a planned intervention to address the valvular disease.7 This case illustrates the complex interplay of these conditions and the heightened risk of uncontrolled AUB if anticoagulation was prescribed.
To the best of our knowledge, this case represents the first documented instance of untreated severe mitral stenosis co-occurring with symptomatic adenomyosis. Adenomyosis is characterized by the growth of endometrial tissue within the muscular wall of the uterus, leading to painful, heavy menstrual bleeding and/or AUB. Studies indicate that the prevalence of adenomyosis in women undergoing hysterectomy for symptoms such as AUB and pelvic pain is around 42%.8 Hysterectomy remains the definitive treatment for adenomyosis, especially when fertility is no longer a concern, as outlined by clinical guidelines.9 However, as mentioned earlier, hysterectomy was deferred due to severe mitral stenosis that could potentially complicate the surgery. Therefore, this case necessitated us to take a unique approach. Acting in the best interest of the patient, we decided to pursue PMBV in the setting of LAA thrombus with a technique of higher atrial puncture to reduce the risk of thromboembolic events during the procedure. After successful PMBV, severe mitral stenosis was resolved, thus permitting the patient to undergo hysterectomy.
In the current case, the patient's severe mitral stenosis and AF with newly diagnosed LAA thrombus with co-occurrence of AUB posed significant challenges. The complexity of the case necessitated an individualized treatment plan that was tailored to the patient's dynamic clinical status and the interplay of her cardiac and gynecologic conditions.
Conclusions
The case discussed herein highlights that clinical guidelines cannot be universally generalized to every scenario, particularly in complex cases in which multiple serious conditions coexist. It emphasizes implementing a tailored approach according to the individual needs of the patient and adjusting treatments to accommodate specific clinical complexities. Such decisions must be made with a transdisciplinary approach, integrating insights and expertise from various medical specialties to ensure comprehensive care. This approach allows for the best possible patient outcomes, demonstrating the critical importance of flexibility and collaboration in health care settings.
Funding Support and Author Disclosures
ChatGPT was utilized for drafting and editing assistance of the manuscript. However, the authors have thoroughly reviewed and revised the content of the manuscript and assume full responsibility for its accuracy and integrity. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and videos, please see the online version of this article.
Appendix
Transesophageal Echocardiography
Transesophageal echocardiography showing a severely dilated left atrium with contrast retention suggestive of thrombus formation within the left atrial appendage.
Cardiac Computed Tomography Scan
Cardiac computed tomography scan illustrating a windsock-type left atrial appendage with an organized thrombus (red arrow).
References
- 1.Nkomo V.T., Gardin J.M., Skelton T.N., Gottdiener J.S., Scott C.G., Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368(9540):1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 2.Simpson M.T., Kachel M., Neely R.C., et al. Rheumatic heart disease in the developing world. Struct Heart. 2023;7(6) doi: 10.1016/j.shj.2023.100219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwentoh I., Henry T., Raiszadeh F., Kurian D., Ayinla R.M. Hidden for years: a positive outcome in the management of severe mitral valve disease. Chest. 2023;164(4):A439–A440. doi: 10.1016/j.chest.2023.07.354. [DOI] [Google Scholar]
- 4.Noubiap J.J., Nyaga U.F., Ndoadoumgue A.L., Nkeck J.R., Ngouo A., Bigna J.J. Meta-analysis of the incidence, prevalence, and correlates of atrial fibrillation in rheumatic heart disease. Glob Heart. 2020;15(1):38. doi: 10.5334/gh.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farman M.T., Sial J.A., Khan N., Rahu Q.A., Tasneem H., Ishaq M. Severe mitral stenosis with atrial fibrillation—a harbinger of thromboembolism. J Pak Med Assoc. 2010;60(6):439–443. [PubMed] [Google Scholar]
- 6.Hodzic E., Granov N. Gigantic thrombus of the left atrium in mitral stenosis. Med Arch. 2017;71(6):449–452. doi: 10.5455/medarh.2017.71.449-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77(4):e25–e197. doi: 10.1016/j.jacc.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 8.Krentel H., De Wilde R.L. Prevalence of adenomyosis in women undergoing hysterectomy for abnormal uterine bleeding, pelvic pain or uterine prolapse—a retrospective cohort study. Ann Med Surg (Lond) 2022;78 doi: 10.1016/j.amsu.2022.103809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lefebvre G., Allaire C., Jeffrey J., et al. SOGC clinical guidelines. Hysterectomy. J Obstet Gynaecol Can. 2002;24(1):37–61. quiz 74-6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transesophageal Echocardiography
Transesophageal echocardiography showing a severely dilated left atrium with contrast retention suggestive of thrombus formation within the left atrial appendage.
Cardiac Computed Tomography Scan
Cardiac computed tomography scan illustrating a windsock-type left atrial appendage with an organized thrombus (red arrow).