Abstract
A 28-year-old woman, gravida 2 para 1, with previously unknown severe rheumatic mitral stenosis presented with progressive dyspnea at 26 weeks of gestation. Percutaneous commissurotomy was considered but was deferred after symptom improvement with beta-blockers and diuretics. Pregnancy complications ensued, requiring preterm delivery. Postpartum percutaneous commissurotomy was successful, highlighting the complexities in managing rheumatic heart disease during pregnancy.
Key Words: mitral stenosis, percutaneous balloon mitral commissurotomy, pregnancy, rheumatic heart disease
Graphical Abstract
History of Presentation
A 28-year-old pregnant woman, gravida 2 para 1, at 26 weeks of gestation was referred to the cardiology outpatient clinic of a tertiary hospital center by her family medicine physician because of progressive dyspnea and abnormal heart sounds. She reported that the dyspnea (NYHA functional class II) began after her previous pregnancy 4 years ago and worsened during the first trimester of her current pregnancy (NYHA functional class IV). On physical examination, the patient weighed 54.5 kg and had a blood pressure of 106/60 mm Hg, heart rate of 84 beats/min, and oxygen saturation of 99%. Cardiac examination revealed a mid-diastolic murmur with an opening snap near S2 in the mitral area.
Learning Objectives
-
•
To understand the interplay between the physiological changes of pregnancy and mitral stenosis.
-
•
To understand the complexities of clinical and procedural therapeutics for rheumatic mitral stenosis during pregnancy.
-
•
To be able to manage rheumatic heart disease in pregnancy, especially when the diagnosis occurs during gestation.
Medical History
The patient had a previous pregnancy (gravida 2 para 1) with no complications. Other than this, she had no prior medical history and denied a history of acute rheumatic fever.
Differential Diagnosis
Other conditions can present with symptoms similar to those of rheumatic mitral stenosis (MS), including peripartum cardiomyopathy, pulmonary embolism, anemia, and infection. However, the specific auscultatory findings and associated symptoms may differ.
Investigations
A 3-dimensional transthoracic echocardiogram revealed trace mitral regurgitation (MR) and severe MS, with a mitral valve area of 1.06 cm2, mean gradient of 12 mm Hg and a systolic pulmonary artery pressure of 70 mm Hg (Figures 1 and 2). Rheumatic alterations were noted in the mitral valve, with commissural fusion, anterior leaflet doming, and restriction of the posterior leaflet (Video 1). The valve anatomy and the Wilkins score were favorable for percutaneous balloon mitral commissurotomy (PBMC).
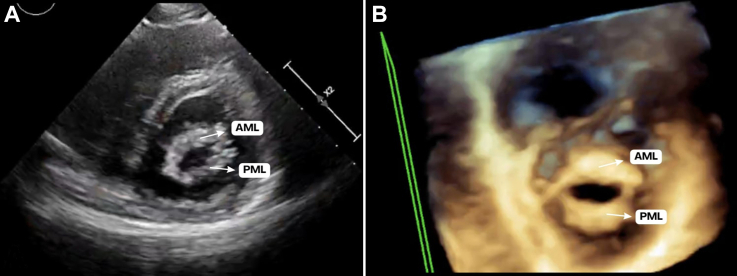
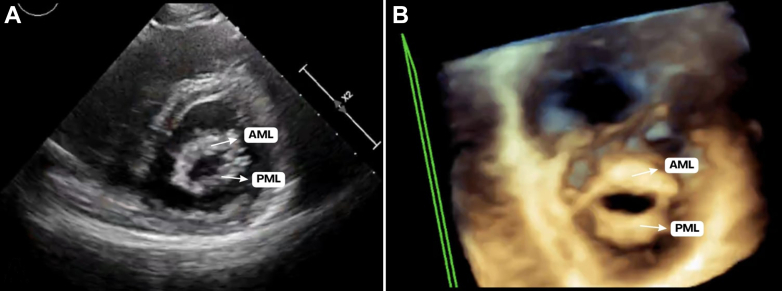
Figure 1.
Mitral Valve at 26 Weeks of Gestation
(A) Transthoracic parasternal short-axis view at the level of the mitral valve. (B) Three-dimensional zoom focused on the mitral valve. Both images exhibit typical rheumatic features, especially a “fish-mouth” mitral valve orifice appearance, increased thickness of the mitral leaflets, and significant commissural fusion. AML = anterior mitral leaflet; PML = posterior mitral leaflet.
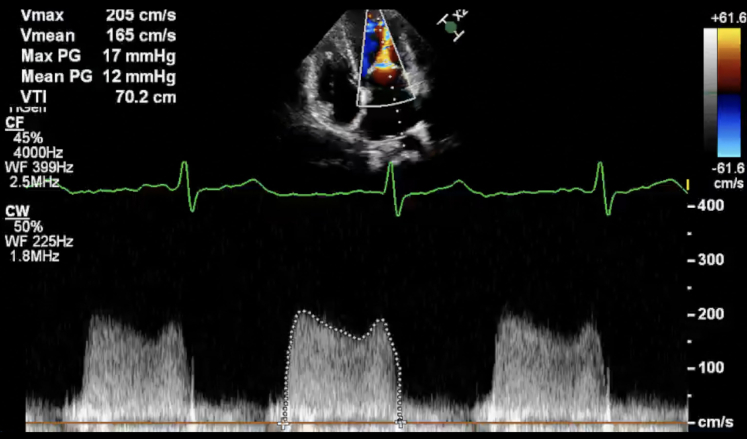
Figure 2.
Transmitral Gradients at 26 Weeks of Gestation
Continuous-wave Doppler reveals severe mitral stenosis with a transmitral mean gradient of 12 mm Hg and peak gradient of 17 mm Hg. Max PG = maximal pressure gradient; Mean PG = mean pressure gradient; Vmax = maximal velocity; Vmean = mean velocity; VTI = velocity–time integral.
Management
The patient was admitted to the hospital by the cardiologist and obstetrician team with the intention of undergoing PBMC. Metoprolol, intravenous furosemide, and supplemental oxygen therapy were administered, and the heart team was ready to perform PBMC urgently if necessary. Her symptoms improved significantly, and the risks and benefits were discussed between the patient, obstetrician, and cardiologist, leading to the decision for conservative treatment. It was agreed to re-evaluate her condition 6 months postpartum for possible PBMC. She stopped prenatal counseling with her family doctor and began follow-up at the high-risk pregnancy clinic with the obstetrician team and was instructed to return for any symptoms suggestive of the need for PBMC.
At a follow-up appointment at 35 weeks and 5 days of gestation, a Doppler ultrasound revealed intrauterine growth restriction, necessitating delivery. She was hospitalized on corticosteroid therapy and underwent a cesarean section. The newborn was healthy with no complications. Six months later, she returned for PBMC. After evaluation, valve anatomy and Wilkins score were favorable for PBMC, and the procedure was performed. The intervention was well tolerated and resulted in symptomatic improvement, with an increase in the mitral valve area to 1.60 cm2 and split of the posteromedial commissure, without an increase in MR severity (Figures 3 and 4).
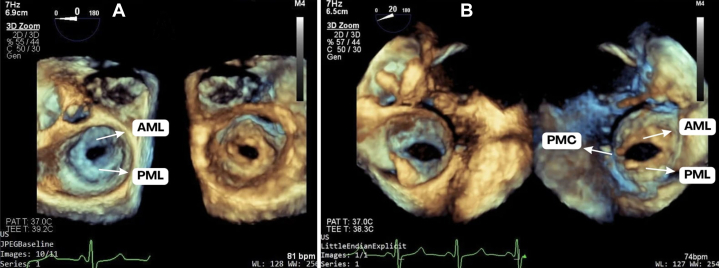
Figure 3.
Mitral Valve Before vs After PBMC at the Transesophageal Echocardiogram
Three-dimensional zoom focused on the mitral valve with mirrored images from the atrial (left side) and ventricle view (right side) for (A) pre-PBMC and (B) post-PBMC with split posteromedial commissure. AML = anterior mitral leaflet; PBMC = percutaneous balloon mitral commissurotomy; PMC = posteromedial commissure; PML = posterior mitral leaflet.
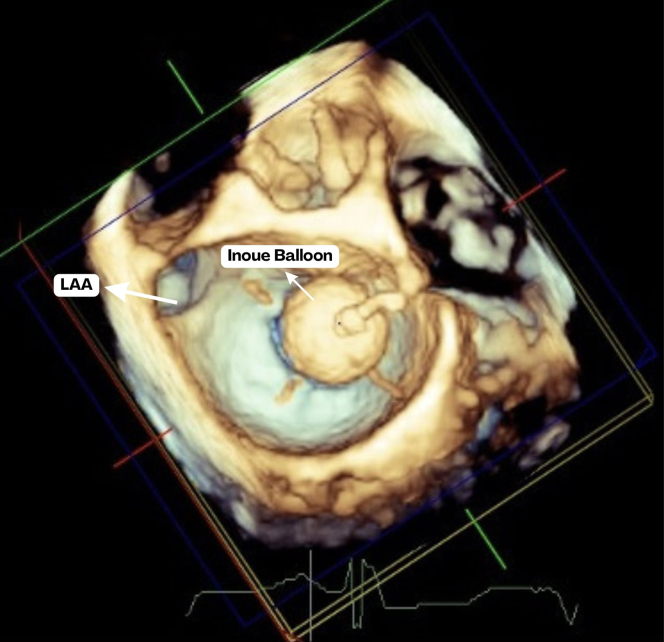
Figure 4.
Inoue balloon Being Inflated During Percutaneous Balloon Mitral Commissurotomy at the Transesophageal Echocardiogram
LAA = left atrial appendage.
Discussion
Rheumatic heart disease (RHD) is the most common acquired valve heart disease seen in pregnancy, affecting predominantly low- and middle-income countries.1 Pregnancy is associated with hemodynamic changes necessary to match the demands of the growing fetus, specifically a progressive increase in cardiac output, heart rate, and blood volume, which peak at 28 to 32 weeks of gestation.2 Rheumatic MS poses an obstruction to left ventricular inflow that increases left atrial and pulmonary pressures, and hemodynamic changes associated with pregnancy exacerbate left heart obstruction, often decompensating previously asymptomatic MS.3 Uteroplacental insufficiency develops secondary to this obstruction, leading to adverse fetal outcomes associated with MS, such as preterm delivery and intrauterine growth restriction, as seen in the case reported.4
Prepregnancy counseling is necessary to evaluate interventions that will allow the patient to better handle the hemodynamic changes of pregnancy, especially in MS. Current guidelines recommend PBMC before pregnancy with favorable valve morphology even in asymptomatic women with severe rheumatic MS (mitral valve area ≤1.5 cm2) who are considering pregnancy.5 PBMC is safe and effective during pregnancy but is contraindicated in cases of significant MR.3,6 In cases of required mitral valve replacement, a decision between bioprosthetic and mechanical valves should be discussed, because bioprosthesis valves have reduced durability in women and mechanical valves require anticoagulation and pose a risk of valve thrombosis.5
In a meta-analysis of observational studies, pregnant women who underwent PBMC demonstrated an overall favorable prognosis. Most unsuccessful procedures were attributed to inappropriate indications for PBMC, particularly due to unfavorable valve anatomy. The overall incidence of de novo or worsened MR postprocedure was nearly 13%, with a restenosis rate of 2.5%, cesarean section rate of 12%, preterm delivery rate of 4%, and low birthweight rate of 5.5%.7 Mortality was reported in 1.5% of cases.7 Although PBMC is considered safe, the risk of acute MR with need for urgent surgical valve replacement should be considered, especially in view of the risk to the health of the fetus.
Medications for the management of RHD are important to prevent adverse maternal outcomes, such as heart failure.5 However, risks and benefits should be weighed considering the potential of injury to the fetus. Diuretics are effective in alleviating volume overload in patients with symptomatic heart failure, but volume reduction should be balanced against the risk of placental hypoperfusion. In patients with MS, left atrial function is necessary to overcome the obstruction of the stenotic orifice. Arrhythmias, especially atrial fibrillation, can decrease the ventricular presystolic volume; therefore, controlling the heart rate is essential to allow proper ventricular filling. Beta-blockers play an important role in easing hemodynamic injury but have been associated with newborn birthweights of ≤100 g, and selective β1 antagonists are preferred to avoid improper uterine relaxation due to β2 receptors.5 Metoprolol is associated with a lower risk of intrauterine growth restriction than atenolol, but the risk should be always considered, as seen in our patient.5 It is important to consider patient compliance with and access to healthcare facilities to ensure optimized medical therapy. Furthermore, conservative management alone does not resolve the left heart obstruction caused by the stenotic orifice. Therefore, potential complications to this injury, such as acute pulmonary edema, should be considered when opting for conservative management.
During labor, the cardiac output increases considerably, driven by pain, anxiety, and uterine contractions.4 After labor, the release of vena cava compression and the increased blood flow from the emptied and contracting uterus further raise the cardiac output.4 This increase in cardiac output during labor can usually be handled by someone with mild to moderate MS without pulmonary hypertension.6 However, for someone with severe MS and an NYHA functional class III or IV with pulmonary hypertension, a cesarean section should be the preferred method of delivery.6 Therefore, in this case, the indicated method of delivery was a cesarean section.
In the case reported, symptom control with medications facilitated the progression of pregnancy beyond 34 weeks of gestation. When properly indicated, PBMC is safe during pregnancy; nevertheless, decision-making including the patient, cardiologist, and obstetrician should always be considered to decide between PBMC and conservative management.
Follow-Up
In the 30-day, 6-month, and 1-year follow-up appointments, the patient remained asymptomatic and continued her regimen of penicillin every 21 days and metoprolol. She expressed no desire for further pregnancies and opted for a copper intrauterine device for contraception. The newborn was fine and developed well.
Conclusions
In pregnant patients with severe rheumatic MS, the risk-to-benefit ratio of undergoing a PBMC should be carefully evaluated against conservative clinical treatment. Thorough prepregnancy counseling is essential to properly diagnose and manage RHD before pregnancy. This case highlights a challenging decision-making scenario and is representative of the common occurrence of RHD diagnosis during pregnancy.
Funding Support and Author Disclosures
Supported in part by the Leducq Foundation PRIMA (Preventing Rheumatic Injury bioMarker Alliance) Network grant 22ARF02. The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental video, please see the online version of this paper.
Appendix
Parasternal long-axis view of the mitral valve at 26 weeks of gestation. Slow-motion (0.5×) reveales significant anterior leaflet doming, restriction of the posterior leaflet, and chordal thickening, consistent with rheumatic mitral stenosis.
References
- 1.Baghel J., Keepanasseril A., Pillai A.A., et al. Prediction of adverse cardiac events in pregnant women with valvular rheumatic heart disease. Heart. 2020;106(18):1400–1406. doi: 10.1136/heartjnl-2020-316648. [DOI] [PubMed] [Google Scholar]
- 2.Sanghavi M., Rutherford J.D. Cardiovascular physiology of pregnancy. Circulation. 2014;130(12):1003–1008. doi: 10.1161/CIRCULATIONAHA.114.009029. [DOI] [PubMed] [Google Scholar]
- 3.van Hagen I.M., Thorne S.A., Taha N., et al. Pregnancy outcomes in women with rheumatic mitral valve disease: results from the Registry of Pregnancy and Cardiac Disease. Circulation. 2018;137(8):806–816. doi: 10.1161/CIRCULATIONAHA.117.032561. [DOI] [PubMed] [Google Scholar]
- 4.Tsiaras S., Poppas A. Mitral valve disease in pregnancy: outcomes and management. Obstet Med. 2009;2(1):6–10. doi: 10.1258/om.2008.080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. 2021;77(4):e25–e197. doi: 10.1016/j.jacc.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 6.Regitz-Zagrosek V., Roos-Hesselink J.W., Bauersachs J., et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39(34):3165–3241. doi: 10.1093/eurheartj/ehy340. [DOI] [PubMed] [Google Scholar]
- 7.Sreerama D., Surana M., Moolchandani K., et al. Percutaneous balloon mitral valvotomy during pregnancy: a systematic review and meta-analysis. Acta Obstet Gynaecol Scand. 2021;100(4):666–675. doi: 10.1111/aogs.14029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Parasternal long-axis view of the mitral valve at 26 weeks of gestation. Slow-motion (0.5×) reveales significant anterior leaflet doming, restriction of the posterior leaflet, and chordal thickening, consistent with rheumatic mitral stenosis.