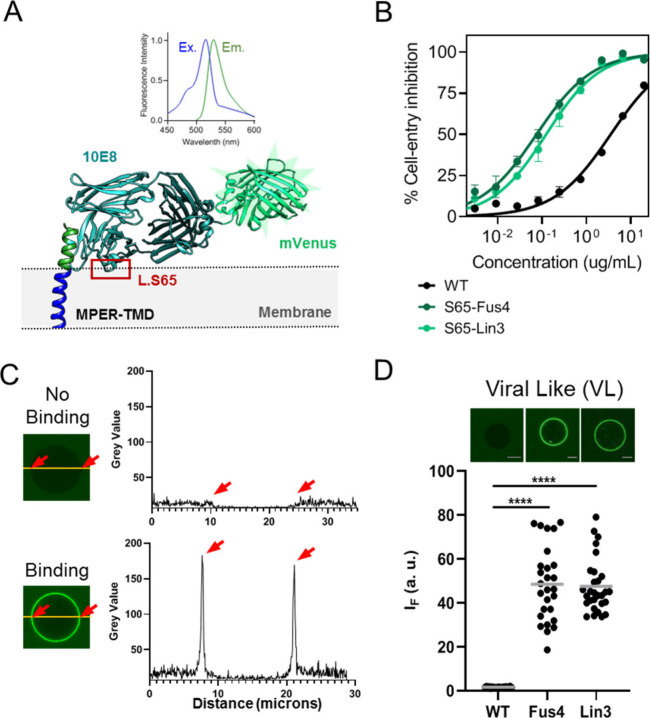
Figure 2.
Binding to single VL vesicles using Fab-mVenus chimeras. A) Structure depicting the 10E8 Fab-mVenus chimera bound to the ctMPER-TMD helix. Excitation/Emission spectra of the purified protein are shown on top, demonstrating the acquisition of the correct tertiary structure by mVenus. B) Cell-entry inhibition activity comparing WT and chemically modified chimeras. Titration values are means ± SD of three independent experiments. C) Quantification of Fab 10E8 binding to single vesicles using the Fab-mVenus chimera. Left panels display confocal microscopy images of single VL vesicles incubated with Fab-mVenus WT or Fab-mVenus-Lin3 (top and bottom, respectively). Traces on the right panels follow the changes in the mVenus fluorescence intensity at the equatorial plane (green label). D) Binding to single VL vesicles comparing the WT chimera with those chemically modified with Fus4 or Lin3. Amount of Fab bound was estimated for each vesicle as the fold increase in mVenus fluorescence intensity over the mean value of the background level (i.e., background intensity normalized to 1). (****p < 0.0001).