Abstract
Background
The evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) persists, giving rise to new variants characterized by mutations in the spike protein. However, public data regarding the virus's evolutionary trend is not widely available after the downgrade of coronavirus disease 2019(COVID-19). Therefore, this study aimed to investigate the applicability of an in-house Sanger-based method for identifying SARS-CoV-2 variants, particularly focusing on newly emerged Omicron variants, and updating the epidemiology of COVID-19 during the 8th wave in Hiroshima Prefecture.
Results
A total of 639 saliva samples of individuals who had tested positive for COVID-19, received from Hiroshima City Medical Association Clinical Laboratory Center between February 01, 2023, and March 12, 2024, were included in the study. SARS-CoV-2 variants were identified in 69.3% (443/639) with the mean viral titer 2 × 106 copies/mL, and high viral titer in Omicron variant XBC.1.6* (5 × 108 copies/mL) using RT-qPCR. By partial Spike gene-based sequencing using the Sanger Sequencing strategy, Omicron sub-lineages XXB.1, BA.5, and EG.1 were identified during different periods. A comprehensive phylogenetic analysis of 7383 SARS-CoV-2 strains retrieved from GISAID, collected in Hiroshima from the onset of the COVID-19 pandemic in early 2020 until July 2024, revealed the dynamic evolution of SARS-CoV-2 variants over time. The study found a similar pattern of variant distribution between the full genomes from GISAID, and the partial genomes obtained from our screening strategy during the same period.
Conclusions
Our study revealed that all SARS-CoV-2 viruses circulated in Hiroshima were Omicron variants and their sub-lineages during the 8th wave outbreak in Hiroshima. Persistent molecular surveillance of SARS-CoV-2 is needed for the decision-making and strategic planning of the public promptly. Our study added evidence for the usefulness of SARS-CoV-2 spike gene partial sequencing-based SARS-CoV-2 variant identification strategy for mass screening and molecular surveillance even though the evolution of newly emerged various SARS-CoV-2 Omicron variants.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12864-024-10973-0.
Keywords: SARS-CoV-2, Variants, Omicron
Background
The coronavirus disease, characterized by its high infectivity and pathogenicity, is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1, 2]. This coronavirus disease 2019(COVID-19) pandemic, which emerged in late 2019 and spread very quickly across the world, has resulted in a total of over 771 million confirmed cases, including over six million fatalities worldwide since the inception of this deadly disease as of November 10th, 2023. There have been over 33 million confirmed cases in Japan and 74,694 reported deaths. Specifically in Hiroshima Prefecture, located in the southwest of Japan’s main island, it was estimated about 816,788 confirmed cases resulted in 1,373 reported deaths [3].
Over time, since the outbreak occurred in the past three years, this disease has evolved, giving rise to various variants that have heightened the global public health threat. These extended infections increase in new mutational variants, which allow the virus to spread quickly or make it resistant to treatments or vaccines. Significantly, alterations in the spike (S) gene of SARS-CoV-2 can influence the virus's ability to enter host cells, its infectivity, transmission dynamics, and the effectiveness of protective immune responses [4]. SARS-CoV-2 variants are circulating and evolving globally due to their propensity for mutation and recombination. Some of them are categorized as variants of concern (VOCs, namely Alpha, Beta, Gamma, Delta, and Omicron) and variants of interest (VOIs namely Epsilon, Zeta, Eta, Theta, Iota, Kappa, Lambda, Mu) based on the risk posed to global public health [5]. Recently, WHO has been actively tracking several SARS-CoV2 variants including three VOIs: XBB.1.5, XBB.1.16, and EG.5, and seven variants under monitoring: BA.2.75, BA.2.86, CH.1.1, XBB, XBB.1.9.1, XBB.1.9.2 and XBB.2.3 [6]. This continuum of virus mutations could challenge public health systems and impact strategies to combat the COVID-19 pandemic.
Due to the decrease in the trend of COVID-19 deaths, COVID-19 hospitalized, insensitive care unit admissions, and the high level of herd immunity to SARS-CoV-2 infection, WHO announced downgrading COVID-19 as no longer constitutes a public health emergency of international concern (PHEIC); however, it does not mean that the global threat by COVID-19 is over as the virus continues to mutate, especially the mutation on the spike gene. After WHO announced the end of the emergency phase of COVID-19 in May 2023, many countries have stopped reporting and integrating COVID-19 into respiratory disease surveillance, as a result, there is limited public data regarding the virus's evolutionary trend. Additionally, it's essential to exercise caution when interpreting the recent decrease in infection rates, as this may be attributed to the reduced in testing, sequencing, and reporting and reporting delays in numerous countries [6].
In our previous reports, we have developed and evaluated a simple and effective SARS-CoV-2 variant identification strategy using SARS-CoV-2 spike gene partial sequencing by the Sanger method which provided a significantly higher variant identification rate and applicable for those saliva samples with low viral titer where next-generation sequencing is challenging [7–10]. After the emergence of various SARS-CoV-2 Omicron variants with a variety of mutations, the applicability of our in-house developed SARS-CoV-2 variants identification strategy is questionable. Therefore, this study aimed to investigate the applicability of in-house developed Sanger-based SARS-CoV-2 variants identification strategy among newly emerged various SARS-CoV-2 Omicron variants and then to update the epidemiology of COVID-19 during the 8th wave in Hiroshima Prefecture.
Methods
Study subjects
Japan has reclassified COVID-19 as “A class 5 disease according to the Infectious Diseases Control law. Because it is at the same level as the seasonal flu, the countermeasure has been changed. All the clinics are equipped to perform the COVID-19 PCR test and report the positive case to local and regional health center. In collaboration with the Hiroshima City Medical Association Clinical Laboratory Center,our study included a total of 639 saliva samples from individuals who had tested positive for COVID-19 polymerase chain reaction (PCR) between February 01, 2023, and March 12, 2024, in Hiroshima. The saliva samples were collected from COVID-19 confirmed cases in all clinics in Hiroshima using the Opt-Out system where the use of the saliva samples for research purposes is notified to all individuals visiting the clinic for COVID-19 testing and every individual has right to defer using their samples in our study. Figure 1 shows the study's workflow and provides insights into the samples' distribution for SARS-CoV-2 mutant screening. All the samples used in this study were rendered anonymous, and no personal information was included.
Fig. 1.
Distribution of the number of samples for SARS-CoV-2 variants screening. The distribution of the number of saliva samples from individuals who had tested positive for COVID-19 received from the Hiroshima City Medical Association Clinical Laboratory Center from February 2023–March 2024 for SARS-CoV-2 variants screening in Hiroshima during the 8th wave
Extraction of nucleic acid and quantification of SARS CoV-2 Virus by qRT-PCR
SARS-CoV-2 RNA was extracted by using the Maxwell® RSC viral total nucleic acid multi-pack kit (Promega, USA). Following the manufacturer’s protocol, 200μL of the saliva samples were added to the tube containing 200μL Lysis buffer, and 20μL Protein Kinase, followed by a 10-min incubation at 56 °C. Subsequently, the sample mixture was introduced into Maxwell® RSC cartridges and run at total nucleic acid extraction protocol. Finally, the nucleic acid products were automatically transferred into elution tubes containing 50µL of RNase water. Then, the SARS-CoV-2 viral titer was quantified by real-time reverse transcription PCR (qRT-PCR) as described previously [7, 9, 10] and the titer was transformed into copies per milliliter (copies/mL).
SARS-CoV-2 Spike gene partial sequencing for SARS-CoV-2 variant screening and identification
The amplification of the SARS-CoV-2 spike gene having 798 base pairs was performed by nested RT-PCR using in-house primer sets as described previously [9, 10]. The outer primer set includes forward primers (22632S:5’GAATCAGCAACTGTGTTGCTG3’, 22659S:5’CTGTCCTATATAATTCCGCATC3’, 22659S-Omi:5’CTG TCCTATATAATCTCGCACC3’) and reverse primers (SP35AS:5’TGA CTAGCTACACTACGTGC3’, SP36AS: 5’TT AGTCTGAGTCTGATAACTAG3’). The inner primer set includes the forward primers (22687S:5’CACTTT TAAGTGTTATGGAGTG3’, 22712S:5’CCTACTAAATTAAATGATCTCTG3’) and reverse primers (SP37AS: 5’GCATATACCTGCACCAATGG3’, SP38 AS:5’TATGTCACACTCATATGAGTTG3’). The amplicons were then visualized by gel electrophoresis and the successfully amplified products were then purified by MinElute® 96UF PCR purification kit (QIAGEN, Germany) and underwent the Sanger sequencing using SeqStudio GENETIC Analyzer (Thermo Fisher Scientific, Applied Biosystems, Foster City, CA, USA) as per the previously described method [10]. The raw data was visualized by GENETYX-MAC (version 21.0.1, GENETYX Corporation, Tokyo, Japan). Then, SARS-CoV-2 variants are identified by the unique checkpoints of base nucleotide mutation in the spike gene as shown in the Supplementary Fig. 1.
Investigation of the dynamic of SARS-CoV-2 evolution during 2020 ~ 2024 by phylogenetic tree analysis of 7383 SARS-CoV-2 strains submitted at GISAID from Hiroshima
A comprehensive phylogenetic analysis was conducted on 7,383 SARS-CoV-2 strains retrieved from the Global Initiative on Sharing All Influenza Data (GISAID) database (https://gisaid.org). These retrieved strains were collected in Hiroshima from the onset of the COVID-19 pandemic in early 2020 through to July 2024, providing a detailed overview of the viral evolution over this period. The phylogenetic tree was constructed by the neighbor-joining method using 70% cut-off value with 1000 bootstrap replication in Molecular Evolutionary Genetics Analysis (MEGA) version 11 [11] to determine the evolutionary relationships and track the emergence and spread of different variants.
Results
SARS-CoV-2 viral titer by variants
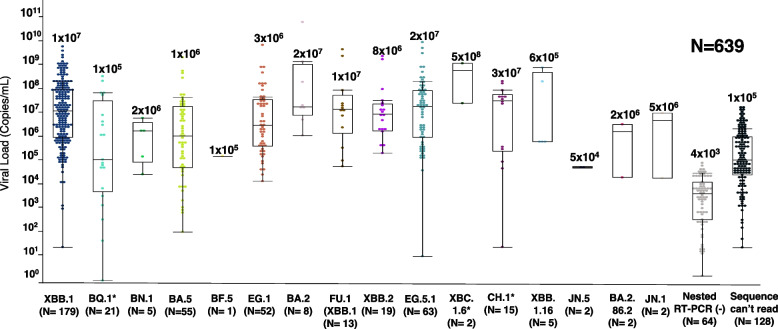
From February 1, 2023, to March 12, 2024, a total of 639 saliva samples received from the Hiroshima City Association Clinical Laboratory Center were included in this study. The viral titer ranged from 5 × 104 to 5 × 108 copies/mL, with an average of 2 × 106 copies/mL. The high viral titer of SARS-CoV-2 was found in Omicron sub lineage XBC.1.6* (mean viral titer: 5 × 108 copies/mL), CH.1* (mean viral titer: 3 × 107 copies/mL), EG.5.1 (mean viral titer: 2 × 107 copies/mL), and BA.2 (mean viral titer: 2 × 107 copies/mL). Of 639 samples, 10% of samples (64/639) were failed to amplify spike gene (mean viral titer 4 × 103 copies/ mL), and 20% of samples (128/639) were failed to sequence (mean viral titer: 1 × 105 copies/mL), and the remaining 443 samples were subjected to variant identification (Fig. 2).
Fig. 2.
The Viral Titer of SARS-CoV-2 stratified by variants. The viral load expressed in copies per milliliter of the SARS-CoV-2 is stratified by each variant. The scattered plot indicates the viral tilter of the detected variants with different colors that represent each specific variant
Amplification and variants identification by Sanger sequencing strategy
Among a total of 639 samples, 93 had a viral titer ≤ 103 copies/mL, 325 had a viral titer from 104 to 106 copies/mL and 221 had a viral titer > 107 copies/mL. Our in-house developed SARS-CoV-2 partial spike gene amplification for variants identification provided the successful amplification rate of 48.3% (45/93), 95.1% (309/325), 100% (221/221) in those samples with viral titer ≤ 103, 104 to 106, and > 107copies/mL, respectively. Excluding the amplicon failed to sequence, the variants were successfully identified in 21.5% (20/93), 66.5% (216/325), and 93.7% (207/221) of those samples with viral titer ≤ 103 and 104 to 106 copies/mL, respectively (Table 1).
Table 1.
The rate of amplification and variant identification among SARS-CoV-2 Omicron variants using Sanger Sequencing Strategy
|
Viral titer (copies/mL) |
N |
Complete amplification n (%) |
Variants identification n (%) |
|---|---|---|---|
| ≤ 103 | 93 | 45 (48.3%) | 20 (21.5%) |
| 104 ~ 106 | 325 | 309 (95.1%) | 216 (66.5%) |
| ≥ 107 | 221 | 221 (100%) | 207 (93.7%) |
| Total | 639 | 575 (90.0%) | 443 (69.3%) |
Distribution of SARS-CoV-2 variants from February 2023 to March 2024 in Hiroshima
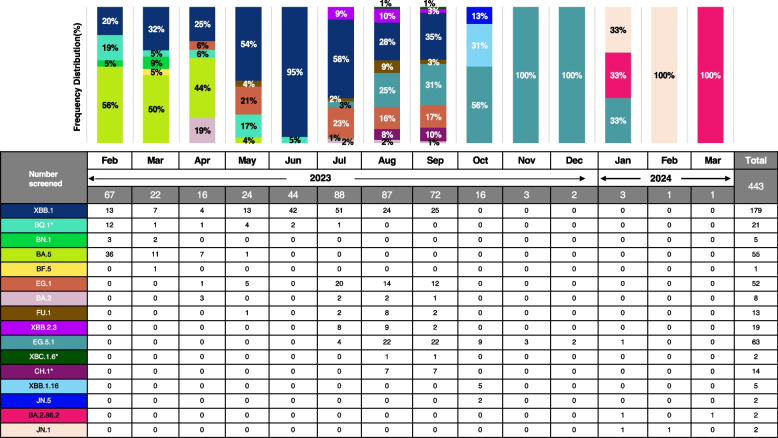
From February to April 2023, the Omicron sub-lineage BA.5 was predominant in Hiroshima, with frequencies of 56%, 50%, and 44%, respectively. The distribution of the Omicron sub-lineage XBB.1 was 20% in February, rising each month to reach 95% in June. However, its prevalence began to decline from July (58%) to August (28%). The Omicron sub-lineage BQ.1 was frequently found in February (19%) and May (17%) 2023. Moreover, Omicron sub-lineage BA.2 was also found in May 2023 (19%). From August 2023 to December 2024, the Omicron sub-lineage EG.5.1 increased in frequency from 25 to 100%. Other Omicron sub-lineages such as XBB.2.3, CH.1, and FU.1 were found in August (10%, 8%, and 9%, respectively) and September 2023 (3%, 10%, and 3%, respectively). The Omicron sub-lineages JN.5 (13%) and XBB.1.16 (31%) were identified in October 2023. Between January and March 2024, 3 types of variants named as EG.5.1, BA.2.86.2 and JN.1 were detected (Fig. 3).
Fig. 3.
Percentage distribution of SARS-CoV-2 variants in Hiroshima by each month. The percentage distribution of the SARS-CoV-2 variants was identified during the screening period from February 2023–March 2024 indicating by each month. Each variant was shown with a specific color
The dynamic of SARS-CoV-2 evolution between March 2020 and July 2024 in Hiroshima
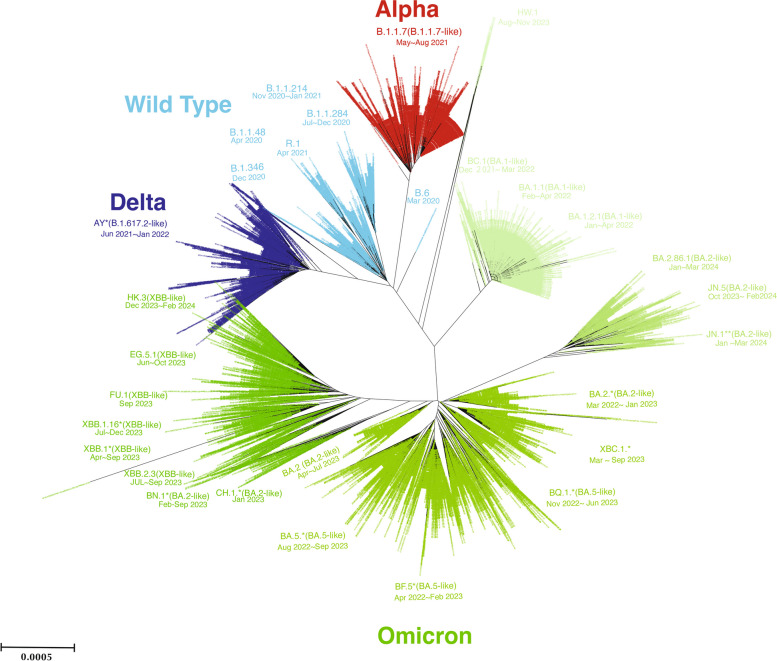
The phylogenetic tree revealed the presence of multiple significant SARS-CoV-2 variants in Hiroshima between March 2020 when the first case of COVID-19 was detected in Hiroshima and July 2024. This analysis included total 7383 SARS-CoV-2 strains retrieved from GISAID. In the early phase of outbreak in Hiroshima, the wild-type strains and various B lineages were dominant until April 2021. Since May 2021, the Alpha variant (B.1.1.7) was dominant followed by the Delta variant (B.1.617.2-like) from June 2021 to January 2022. Both the Alpha and Delta variants exhibit the less branching in the phylogenetic tree. Since December 2021, the Omicron variant was found in Hiroshima when BA.1. was predominant followed by BA.2, BA.5, BF.5, BQ.1 in the year 2022 and early 2023. The other Omicron variants sub lineages XBC.1, XBB.1 and XBB.2 series, EG.5, FU.1, BA.2.86, etc. emerged in year 2023 which were then replaced by JN.1, JN.5, HK.3 (XBB-like) in year 2024 in Hiroshima. Over time, the emergence of Omicron variants was observed, with several sub-lineages such as BA.2, BA.5, and XBB becoming prevalent. Each variant's timeframe of predominance was documented, indicating shifts in the dominant strains across different waves of the pandemic (Fig. 4).
Fig. 4.
The dynamic of SARS-CoV-2 variant evolutions between March 2020 and July 2024 in Hiroshima. The comprehensive phylogenetic tree analysis illustrates the dynamic of SARS-CoV-2 variants in Hiroshima. A total of 7383 SARS-CoV-2 full genomes submitted from Hiroshima to GISAID from March 2020 to July 2024, were retrieved and included in the analysis. The phylogenetic tree was constructed by the neighbor-joining method using MEGA.11 software
Discussion
SARS-CoV-2 continues its global and regional spread. In the 28-day interval from September to October 22, 2023, COVID-19 cases were reported by 93 countries, and 38 countries reported COVID-19-related deaths. According to the available data, there has been a decline in the number of reported cases and deaths during these 28 days, showing a decrease of 42% in new cases and 43% in new deaths compared to the preceding 28 days (from August 28 to September 24, 2023). It is important to note that reported cases may not accurately reflect infection rates due to reduced testing and sequencing, along with reporting delays in many countries [6].
This study is a continuum of SARS-CoV-2 variant screening conducted since the beginning of the COVID-19 epidemic in Hiroshima Prefecture. Together with the Hiroshima City Association Clinical Laboratory Center , a total of 639 saliva samples from February 1, 2023, to March 12, 2024, were included for the SARS-CoV-2 variants screening, using the Sanger Sequencing Strategy with the modified in-house primer set validated in our previous study [10]. Among a total of 639 samples, both the amplification and variants identification rate were increased by viral titers, and it sustainably provided the amplification rate at 48.3% in low viral titer ≤ 103 copies/mL but considerably decreased from 89.7% in our previous report [8]. However, our Sanger sequencing-based SARS-CoV-2 variants identification strategy is sustainably able to apply after evolution of various SARS-CoV-2 Omicron variants. Additionally, our method still could identify new variants despite the lower viral titer (≤ 103 copies/mL).
Based on our previous report, from the 3rd to 6th wave in Hiroshima Prefecture, we found that each outbreak was linked to the dominance of a specific SARS-CoV-2 variant: the R.1 variant in the 3rd wave, the Alpha variant (B.1.1.7) in the 4th wave, the Delta variant (B.1.617.2) in the 5th wave, and the Omicron variant (B.1.1.529) in the 6th wave [10]. Our current study revealed that all SARS-CoV-2 circulated in Hiroshima during the 8th outbreak of the COVID-19 infection are Omicron variants (XBB.1, BQ.1*, BN.1, BA.5, BF.5, EG.1, BA.2, FU.1, XBB.2.3, EG.5.1, XBC.1.6*, CH.1*, XBB.1.16, JN.5, BA.2.86.2, and JN.1). Specifically, Omicron (BA.5) was predominant from February to April 2023 while from May to September 2023, Omicron (XBB.1) was the most detected case. Other SARS-CoV-2 Omicron sub-lineages BQ.1, BA.2, and EG.5 were increasingly found from May to August 2023, and later the other SARS-CoV-2 Omicron sub-lineages XBB.2.3, CH.1*, FU.1, XBB.1.16, JN.5, BA.2.86.2, and JN.1 were also circulated in August 2023 to March 2024.
We also analyzed the phylogenetic tree to understand the evolution and distribution of SARS-CoV-2 variants in Hiroshima, after retrieving all the submitted SARS-CoV-2 full genomes from Hiroshima Prefecture at GISAID. The analysis highlighted the dynamic nature of SARS-CoV-2 evolution and the successive waves of different variants affecting Hiroshima. The initial spread of wild-type strains and early variants like Alpha and Delta was followed by the more transmissible Omicron variants, reflecting global trends. The phylogenetic revealed difference branching patterns among SARS-CoV-2 wild type, Alpha variant, Delta variant and the Omicron variant. All the wild type, the Alpha and the Delta exhibit the less branching pattern, with the shortness of branch and consisting of large number of cases in the same branch indicates the less genetic variation and mutation during the early phase of outbreak in Hiroshima. Since the Omicron variant emerged alongside with subsequent evolution of multiple Omicron sub lineages within a short period indicates the change in mutation rate of SARS-CoV-2 and rapid evolution. Thus, the Omicron variants in the phylogenetic tree provided the multiple branching and longer branches carrying cluster cases. The Omicron era is obviously differed from the previously emerged its ancestor and the rapid emergence of Omicron sub-lineages such as BA.2, BA.5, and XBB indicates ongoing viral adaptation and immune escape, posing challenges for public health responses. Continuous monitoring and sequencing are crucial for identifying new variants and informing vaccination and mitigation strategies. This study underscores the importance of global data sharing and collaborative efforts in tracking and combating the COVID-19 pandemic. Therefore, it is suggested that our Sanger-based variant identification strategy is effective in identifying the newly emerged various SARS-CoV-2 Omicron variants in Hiroshima in addition to previously emerged Alpha, Delta and other variants and is subjected as an alternative variants screening strategy to highly cost gold standard next generation sequencing.
In November 2021, WHO declared that the world encountered a new variant of concern known as the Omicron variant (B.1.1.529), which was first reported in South Africa [12] and continues to spread worldwide. Over the past few months, various lineages of the Omicron SARS-CoV-2 variant have been detected worldwide, including the BA.1 and BA.2 subvariants. Presently, the dominant strain in the United States is the BA.2.12.1 subvariant, while in South Africa, BA.4 and BA.5 hold prominence. Additionally, the Centaurus BA2.75 and BA2.75.2 subvariants have also been identified [13]. As of February 2022, the prevalence of the Omicron variant has led to a rise in COVID-19 cases in Japan [14]. Omicron variant has high transmissibility and the capacity to evade immunity leading to its rapidly replacing Delta as the predominant strain globally [15]. Regarding the severity, previous studies reported that Omicron variants are less likely to develop severe clinical outcomes compared to other previous variants [16–18]. Moreover, prior studies found that patients infected with Omicron were significantly less likely to require hospitalization or ICU care [19, 20]. Nonetheless, there is a possibility that the pathogenicity of the Omicron variant might be underestimated due to numerous factors including the increasing levels of herd immunity resulting from prior infections, high vaccination coverage, and other health conditions [16, 21, 22]. Our study found that the Omicron variant was dominant compared to other previous variants.
SARS-CoV-2 has acquired many novel mutations since the onset of the COVID-19 pandemic, as a result, an increase in adaptive diagnostic methods for variant screening is required. Generally, to confirm a specific SARS-CoV-2 variant, Whole Genome Sequencing (WGS), or at least complete or partial S-gene sequencing, is the more reliable method for identifying the SARS-CoV-2 variant and its characteristics. Other methods, such as nucleic acid amplification technique (NAAT)-based assays for diagnostic screening, have been developed to enable early detection and pre-screening for estimating the prevalence of Variants of Concern (VOCs), Variants of Interest (VOIs), and Variants Under Monitoring (VUM). While some of these methods can accurately identify different variants, others may require confirmation through sequencing, focusing on at least the complete or partial S-gene genomic region in a subset of samples [23]. In our study, we used a Sanger sequencing method, which is low-cost, compared to other methods, to identify the SARS-CoV-2 variants that were circulating in Hiroshima Prefecture.
Since the beginning of the COVID-19 epidemic in Japan, we studied the molecular characteristics, and the mutation pattern of the SARS-CoV-2 virus circulated in Hiroshima [7]. At the time of the evolution of SARS-CoV-2 from the Asian clade into the European clade, we developed the in-house primer set to differentiate between the Asian clade and the European clade, using the in-house developed identification method [7]. Once WHO notified SARS-CoV-2 VOCs, VOIs, and VUMs after the emergence of SARS-CoV-2 Alpha, Beta, and Gamma variants, we also developed and reported in-house strategy for the identification of SARS-CoV-2 variants using SARS-CoV-2 Spike gene partial genome sequencing by Sanger method [9], which proved that the identification was 100% agreed to gold standard Next Generation Sequencing (NGS) and the SARS-CoV-2 amplification rate was increased to 89.7% compared to NGS (19.2%) among the samples with low viral load < 103 copies/mL [8]. Therefore, our in-house developed SARS-CoV-2 spike gene partial genome sequencing-based variant identification helped increase the variant identification rate and promoted the molecular surveillance of SARS-CoV-2 during the public health emergency and was practically applicable for mass screening.
Conclusions
Our study revealed that all SARS-CoV-2 viruses circulated in Hiroshima were Omicron variants and their sub-lineages during the 8th wave outbreak in Hiroshima. Our study yielded the molecular surveillance of SARS-CoV-2 though the Japanese MHLW reclassified the SARS-CoV-2 as class 5 infectious disease. Our in-house developed SARS-CoV-2 variants identification consistently provided a high amplification rate even though in low viral titer ≤ 103 copies/mL. Persistent molecular surveillance of SARS-CoV-2 is needed for the decision-making and strategic planning to the public promptly and our study added evidence for the usefulness of SARS-CoV-2 spike gene partial gene sequencing-based SARS-CoV-2 variant identification strategy for mass screening and molecular surveillance.
Supplementary Information
Acknowledgements
The authors acknowledge the Hiroshima City Medical Association Clinical Laboratory Center for the valuable support of this study and their support on the arrangement of sample collection. The authors also wish to acknowledge all of those who contributed and deposited sequences at GISAID and GenBank.
Authors’ contributions
JT, KK, and KT designed the study and methodology. HS, TY, and JT supervised, manage the sample collection and field work. CC, KK, SO, ZP, GAA contributed for the laboratory work. CC, KK, AS, and TA contributed for analysis. CC and KK developed the first draft. JT, KK, and KT provided the critical revision. All authors review the manuscript.
Funding
This research was supported by Hiroshima Prefecture Government-academia collaboration project funding, the Japan Agency for Medical Research and Development (AMED) under Grant Numbers JP20fk0108453, JP21fk0108550 and JP24fk0108706. The funder had no role in the study design, data collection, analysis, interpretation, or manuscript preparation.
Data availability
All data used in this study are fully described in the figures and tables. The sequence data were deposited at GenBank (https://www.ncbi.nlm.nih.gov/genbank/) with the accession number LC829654-LC830158 or can be obtained from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
In this study, the Opt-Out consent method was employed. All patients visiting the clinic for COVID-19 testing were informed about the study’s purpose and the potential use of their saliva specimen. They were given the option to decline participation or withdraw their sample if they tested positive. All samples were deidentified and no personal information was from the clinic. Consequently, the requirement for written informed consent was waived by the Ethical Committee of Hiroshima University. This study was approved by the Ethics Committee of Hiroshima University (E2020-2124–7). All the study procedures strictly adhered to the guidelines and the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ko Ko, Email: kko@hiroshima-u.ac.jp.
Junko Tanaka, Email: jun-tanaka@hiroshima-u.ac.jp.
References
- 1.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO). Fact sheets: Coronavirus disease (COVID-19). Available from: https://www.who.int/news-room/fact-sheets/detail/coronavirus-disease-(covid-19). Accessed 11 Oct 2023.
- 3.World Health Organization (WHO). Coronavirus (COVID-19) Dashboard. 2023. Available from: https://covid19.who.int/. Accessed 4 Feb 2024.
- 4.Li Q, Wu J, Nie J, Zhang L, Hao H, Liu S, et al. The Impact of Mutations in SARS-CoV-2 Spike on Viral Infectivity and Antigenicity. Cell. 2020;182(5):1284–94.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO). Tracking SARS-CoV-2 variants. 2023. Available from: https://www.who.int/activities/tracking-SARS-CoV-2-variants . Accessed 15 Sep 2023.
- 6.World Health Organization (WHO). COVID-19 Epidemiological Update - 29 September 2023. Available from: https://www.who.int/publications/m/item/covid-19-epidemiological-update---29-september-2023 . Accessed 02 Oct 2023.
- 7.Ko K, Nagashima S, E B, Ouoba S, Akita T, Sugiyama A, et al. Molecular characterization and the mutation pattern of SARS-CoV-2 during first and second wave outbreaks in Hiroshima, Japan. PLoS One. 2021;16(2):e0246383. [DOI] [PMC free article] [PubMed]
- 8.Ko K, Takahashi K, Ito N, Sugiyama A, Nagashima S, Miwata K, et al. Despite low viral titer in saliva samples, Sanger-based SARS-CoV-2 spike gene sequencing is highly applicable for the variant identification. BMC Med Genomics. 2023;16(1):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ko K, Takahashi K, Nagashima S, E B, Ouoba S, Hussain MRA, et al. Mass screening of SARS-CoV-2 variants using Sanger Sequencing Strategy in Hiroshima, Japan. Sci Rep. 2022;12(1):2419. [DOI] [PMC free article] [PubMed]
- 10.Ko K, Takahashi K, Nagashima S, E B, Ouoba S, Takafuta T, et al. Exercising the Sanger Sequencing Strategy for variants screening and full-length genome of SARS-CoV-2 virus during Alpha, Delta, and Omicron outbreaks in Hiroshima. Viruses. 2022;14(4):720. [DOI] [PMC free article] [PubMed]
- 11.Tamura K, Stecher G, Kumar S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol Biol Evol. 2021;38(7):3022–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. One year since the emergence of COVID-19 virus variantnOmicron. Available from: https://www.who.int/news-room/feature-stories/detail/one-year-since-the-emergence-of-omicron. Accessed 13 Nov 2023.
- 13.Sabbatucci M, Vitiello A, Clemente S, Zovi A, Boccellino M, Ferrara F, et al. Omicron variant evolution on vaccines and monoclonal antibodies. Inflammopharmacology. 2023;31(4):1779–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ren Z, Nishimura M, Tjan LH, Furukawa K, Kurahashi Y, Sutandhio S, et al. Large-scale serosurveillance of COVID-19 in Japan: acquisition of neutralizing antibodies for Delta but not for Omicron and requirement of booster vaccination to overcome the Omicron’s outbreak. PLoS ONE. 2022;17(4): e0266270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan Y, Li X, Zhang L, Wan S, Zhang L, Zhou F. SARS-CoV-2 Omicron variant: recent progress and future perspectives. Signal Transduct Target Ther. 2022;7(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arabi M, Al-Najjar Y, Mhaimeed N, Salameh MA, Paul P, AlAnni J, et al. Severity of the Omicron SARS-CoV-2 variant compared with the previous lineages: a systematic review. J Cell Mol Med. 2023;27(11):1443–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson AG, Amin AB, Ali AR, Hoots B, Cadwell BL, Arora S, et al. COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of Delta and Omicron variant emergence - 25 U.S. Jurisdictions, April 4-December 25, 2021. MMWR Morb Mortal Wkly Rep. 2022;71(4):132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Goethem N, Chung PYJ, Meurisse M, Vandromme M, De Mot L, Brondeel R, et al. Clinical Severity of SARS-CoV-2 Omicron Variant Compared with Delta among Hospitalized COVID-19 Patients in Belgium during Autumn and Winter Season 2021–2022. Viruses. 2022;14(6):1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdullah F, Myers J, Basu D, Tintinger G, Ueckermann V, Mathebula M, et al. Decreased severity of disease during the first global omicron variant covid-19 outbreak in a large hospital in tshwane, south africa. Int J Infect Dis. 2022;116:38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esper FP, Adhikari TM, Tu ZJ, Cheng YW, El-Haddad K, Farkas DH, et al. Alpha to Omicron: Disease Severity and Clinical Outcomes of Major SARS-CoV-2 Variants. J Infect Dis. 2023;227(3):344–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhattacharyya RP, Hanage WP. Challenges in Inferring Intrinsic Severity of the SARS-CoV-2 Omicron Variant. N Engl J Med. 2022;386(7): e14. [DOI] [PubMed] [Google Scholar]
- 22.Nealon J, Cowling BJ. Omicron severity: milder but not mild. Lancet. 2022;399(10323):412–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.European Centre for Disease Prevention and Control, Europe WHORO for methods for the detection and characterisation of SARS-CoV-2 variants–second update.21 June 2022. Available from: https://iris.who.int/bitstream/handle/10665/360875/WHO-EURO-2022-2148-41903-65545-eng.pdf?sequence=1. Accessed 20 Feb 2024.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this study are fully described in the figures and tables. The sequence data were deposited at GenBank (https://www.ncbi.nlm.nih.gov/genbank/) with the accession number LC829654-LC830158 or can be obtained from the corresponding author upon reasonable request.