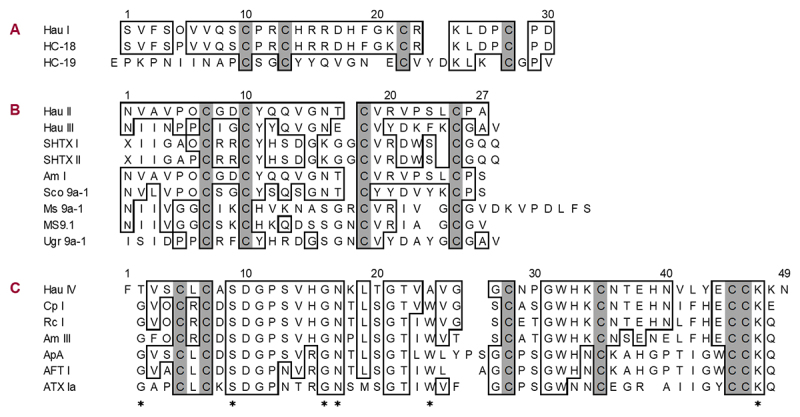
Figure 3. Amino acid sequence alignment of Hau I to Hau IV with analogous sea anemone peptide toxins. (A) Alignment of Hau I with two peptide toxins (HC-18 and HC-19) detected in Heteractis crispa [25]. Cysteine residues are shaded, a hydroxy-Pro residue is denoted by “O”, and identical residues with Hau I are boxed. Note that the only difference between Hau I and HC-18 at the fifth residue (hydroxy-Pro in Hau I and Pro in HC-18) is due to a post-translational modification; the two are completely identical. (B) Alignment of Hau II and III with members of the BBH family. SHTX I and II (type 4 potassium channel toxins) from Stichodactyla haddoni [16]; Am I (possibly sodium channel toxin) from Antheopsis maculata [31]; Sco 9a-1 (AnmTX Sco 9a-1; anti-inflammatory peptide) from Stomphia coccinea [33]; Ms 9a-1 (τ-AnmTX MS 9a-1; modulator of transient receptor potential ankyrin-repeat 1 [TRPA1]) and MS9.1 (AnmTX Ms 9a-2; possibly modulator of TRPA1) from Metridium senile [34]; Ugr 9a-1 (π-AnmTX Ugr 9a-1; acid-sensing ion channel 3 [ASIC3] inhibitor) from Urticina grebelnyi [35]. Cysteine residues are shaded, hydroxy-Pro residues are denoted by “O”, and identical residues with Hau II are boxed. (C) Alignment of Hau IV with type 1 sodium channel toxins. Cp I from Condylactis passiflora [36]; Rc I from Radianthus crispus [37]; Am III from Antheopsis maculata [31]; ApA from Anthopleura xanthogrammica [38]; AFT I from Anthopleura fuscoviridis [39]; ATX Ia from Anemonia sulcata [40]. Cysteine residues are shaded, hydroxy-Pro residues are denoted by “O”, and identical residues with Hau IV are boxed. Asterisks under the sequence of ATX Ia represent the residues typical of only type 1 toxins.