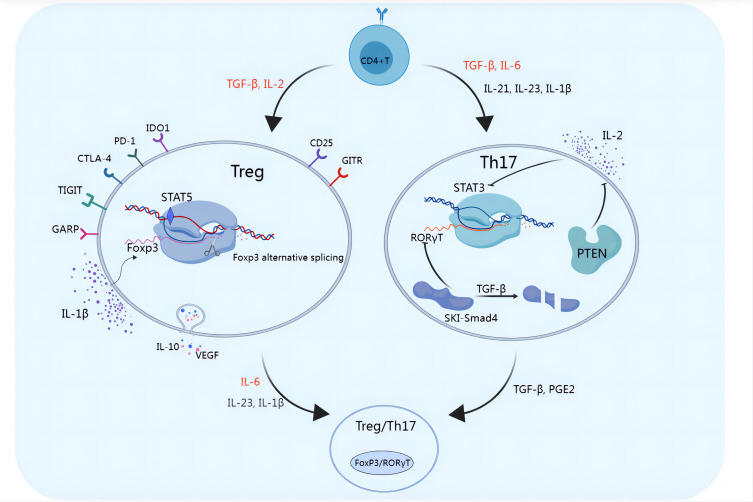
Figure 2.
Functional plasticity of Tregs and Th17 cells. Multiple molecules can affect the functional plasticity of Tregs and Th17 cells. TGF-β and IL-2 induce the differentiation of Tregs, which exert immunosuppressive functions and promote immune escape through the secretion of inhibitory cytokines such as IL-10 and VEGF or through the cell-mediated engagement of inhibitory checkpoint molecules such as GITR, PD-1, CTLA-4, TIGIT, CD25, IDO1, and GARP. IL-1β induces alternative splicing of FoxP3, inhibits Treg cell differentiation, and promotes IL-17 production. RORγt is a key transcription factor in Th17 cell development. The SKI-SMAD4 complex inhibits RORγt, in which the SKI protein inhibits acetylation of the Rorc site. TGF-β was shown to modulate the SKI-SMAD4 complex, and in the presence of TGF-β, SKI is degraded, allowing RORγt to be expressed in CD4+ T cells and ultimately driving Th17 cell differentiation. IL-2 induces STAT5, reduces STAT3 binding, and inhibits Th17 differentiation. PTEN in Th17 cells inhibits the IL-2 signaling pathway, reduces STAT5 and Treg differentiation, and upregulates STAT3. When FoxP3+ Tregs are exposed to IL-6 with or without IL-1β and IL-23, FoxP3 is downregulated, which promotes the expression of Th17 genes, including IL-17, IL-22, IL-23R, and RORγt. TGF-β and PGE2 can also induce Th17-to-Treg cell conversion.