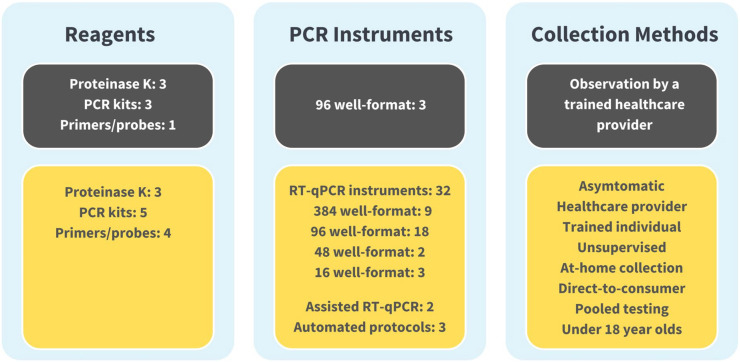
Figure 3.
The SalivaDirect Emergency Use Authorization (EUA), now and then. For broad adoption, we expanded our original EUA protocol (shown in grey boxes)l to include a wider range of workflows ( Figure 1 ) and polymerase chain reaction (PCR) instruments (shown in yellow boxes). By expanding our protocol to include equipment and supplies commonly found in labs, labs could adopt the SalivaDirect PCR protocol and operate under our EUA without significant financial investments in new equipment. Being able to source reagents from multiple suppliers helped to overcome supply chain disruptions while providing options for pricing. Broad collection methods facilitated testing options, permitting sample collection from the clinic to schools and workplaces to the home setting.