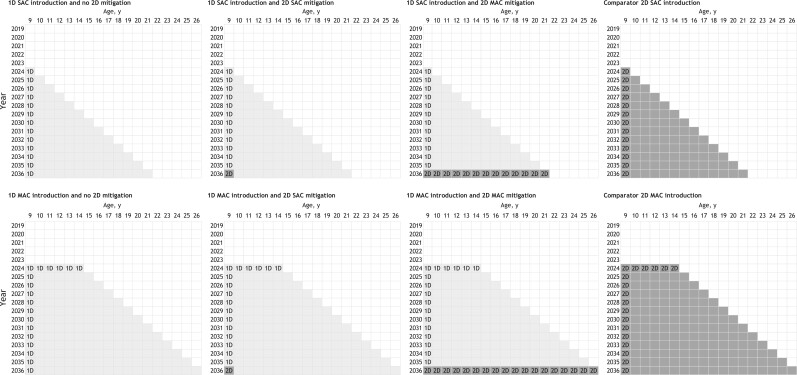
Figure 1.
Primary analysis scenarios contextualized for new adopter countries that consider de novo introduction of a 1-dose human papillomavirus (HPV) vaccination program in 2024 assuming a 1-dose HPV vaccine would provide an average of 30 years of protection prompting a switch to a 2-dose program in 2036 (ie, the 30-year reporting timeline for the Costa Rica Vaccine Trial). Program introduction and 2-dose mitigation vary by the eligible age group(s), that is, single-age cohort age 9 years (y) or additionally providing multi-age cohort vaccination approach. One-dose scenarios ranged from 1-dose single-age cohort introduction in year 2024 with no 2-dose mitigation (top left panel) to 1-dose multi-age cohort introduction (ages 9-14 years) in 2024 with a switch to a 2-dose multi-age cohort mitigation in 2036 (age 9-26 years) (bottom row, panel second from the right). 1D = 1-dose; 2D = 2-dose; MAC = multi-age cohort; SAC = single-age cohort.